- lauraclark849
- Jul 11, 2025
- 20 min read
Updated: Jul 14, 2025
Technical Review Article | Open Access | Published 11th of July 2025
Transethosomal Hydrogel: A Novel Nanocarrier Approach for Enhanced Psoriasis Treatment
Jayesh Mutha, Sweta Srivastava Koka,¹* Sumeet Dwivedi, Ravi Sharma, Ashish Gupta, Pravin Kumar Sharma and G. N. Darwhekar
EJPPS | 302 (2025) | https://doi.org/10.37521/ejpps30214
Abstract
A severe autoimmune condition of skin, psoriasis has major effects on both the body and the mind. The biological absorption and medication penetration of traditional therapies are frequently problematic. Transethosomal hydrogels are investigated in this work as a potential medication delivery method based on nanocarriers for improved psoriasis treatment. Transethosomes provide enhanced skin permeability, enhanced drug stability, and regulated drug release by fusing the advantages of ethosomes and transferosomes. Through the use of ultra-deformable phospholipid structures, these vesicular carriers improve transdermal delivery, allowing for greater bioavailability and deeper skin penetration of medicinal substances. The preparation, benefits, and characterisation methods of transethosomal hydrogels that are highlighted in the paper, along with their patient-friendly uses and sustained-release characteristics. Transethosomal hydrogels provide better pharmacological results, prolonged continuation, and enhanced effectiveness in encapsulation in comparison to conventional dermal therapies. By offering an informal, efficient, and patient-compliant treatment, this study highlights the promise of transethosomal hydrogel-based therapeutics in dermatological care, especially for managing symptoms of psoriasis. To guarantee that this exciting invention is widely adopted, future investigations should concentrate on clinical validation, regulatory approval, and commercialisation.
Keywords: Psoriasis, Transethosomal, Hydrogels, Drug stability, Skin permeability
Introduction
Psoriasis
Behcet (1935) referred to psoriasis (derived from the Greek word psora, which means itching) as "the antidote to a dermatologist's ego," which is still somewhat applicable but far from accurate. Psoriasis is an inflammatory skin condition that can cause severe disfigurement and affects 2% of persons in the UK. Psoriasis's immunological and genetic origins are widely known. A number of novel biological treatments for psoriasis have been developed as a result of this increased understanding, however others have also made contributions. Tumour necrosis factor, for example, is essential to the illness, as seen by the tremendous efficacy of tumour necrosis factor blockers. Furthermore, there is mounting proof that psoriasis is more than just a skin disorder. Epidemiological studies show that people with psoriasis have a higher annualized death rate, particularly for cancer and heart disease. Clinically significant psychological and psychiatric comorbidities are common and treatable. This article summarises all of this and discusses its applications.¹⁻²
The human genome contains at least nine different areas linked to psoriasis. The largest genetic risk factor for psoriasis is PSORS-1, an area of the major histocompatibility complex on chromosome 6p2, which accounts for up to 50% of the condition's hereditary load, despite the fact that the precise gene causing it has not yet been identified. Since many of the linked regions are also found in other autoimmune and inflammatory diseases such atopic dermatitis, multiple sclerosis, inflammatory bowel disease, and type1diabetes, many common complex genetic disorders are brought on by similar pathways.¹

Psoriasis is usually easy to diagnose and manifests as skin rash, nail involvement, and joint sickness. Sometimes patients have atypical skin lesions that must be differentiated from general skin symptoms such as mycosis fungoides, discoid lupus, tinea, scalp scaling, solitary flexural erythema, or genital lesions. A general body check can reveal important diagnostic characteristics that were previously unknown, and in rare instances a skin biopsy may be required. The most prevalent morphological variations of chronic plaque psoriasis are guttate psoriasis, flexural or "inverse" forms (folds of the body), sebopsoriasis, erythrodermic psoriasis (redness and scaling of the entire body), and pustular psoriasis (localized or generalized palmar plantar disease) (psoriasis vulgaris, figs. 1 and 2). The various types can occasionally occur concurrently or at different times.⁵⁻⁸
Clinical Effects
Psoriasis has a heavy psychological cost because it is chronic and incurable. The quality-of-life reductions associated with major illnesses like diabetes, cancer, and heart disease are similar for both hospitalized and primary care psoriasis patients.¹⁻³ Its social, psychological, and functional components all contribute to a reduced standard of living. Morbidity is influenced by a number of factors, including arthritis, treatment-related issues (mess, odour, inconvenience, time), skin-specific symptoms (e.g., persistent itching, bleeding, scaling, nail disease), and the effects of having a highly visible, deformable skin condition (difficulty finding work, low self-esteem, and relationship issues).² Even for individuals with little involvement (less than three palm regions), psoriasis has a big impact on people's lives. Patients who are more ill are likely to have a two- or three-times higher chance of dying from cardiovascular disease. Several interrelated factors will determine this risk (see box 1). A higher risk of various cancers has been identified in numerous hospital and community studies⁶⁻¹⁰, and the risk in patients with severe illness has been found to be similar to that of organ transplant recipients, with notable increases observed for non-melanoma skin cancer and lymphoma. The significance of some recognized confounders, such as concomitant immunosuppressive and phototherapy treatment, alcohol usage, and smoking, is unknown.

Transethosomes
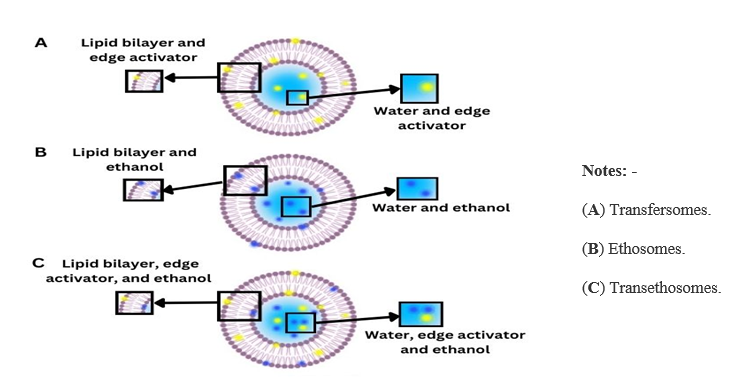
In 1997, ethosomes, a novel type of ultra deformable vesicle (UDV), were developed¹¹. Because of their remarkable elastic properties and size, which ranges between 150 and 200 nm, they are commonly referred to as elastic nanovesicles. These ethosomal systems consist of water and a significant quantity of ethanol-based phospholipid vesicles¹². Phospholipids can be used in amounts between 0.5% and 10% and can be derived from natural, semisynthetic, or synthetic sources, such as soybean and egg. Common phospholipids include phosphatidylcholine, hydrogenated phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol¹³. Ethanol, which makes about 20% to 45% of the mixture, effectively enhances skin absorption¹⁴. This chemical increases the permeability of cell membranes and the fluidity of lipid bilayers by interacting with the polar head groups of stratums corneum (SC) lipid molecules and lowering their melting point¹⁵,¹⁶. Ethosomes are typically categorized as a type of transferosome; the primary distinction is that the ethanol in the formulation evaporates when applied to the skin in nonocclusive conditions. On the other hand, the edge-active molecules, which are typically surfactants, remain on the skin's surface for transferosomes when the water in the formulation evaporates. The precise mechanism by which ethosomes enter the skin is yet unknown. Phospholipids and ethanol are believed to cooperate to facilitate deeper drug distribution and skin layer penetration. Ethosomes' elastic qualities appear to be the primary cause of their improved skin delivery efficacy over alcoholic solutions or traditional liposomes, particularly when it comes to rupturing the lipophilic SC barrier¹⁷,¹⁸,¹⁹. Transethosomes, lipid vesicles derived from both ethosomes and transferosomes, were first discovered by Song et al. in 2012¹⁹. Their ability to combine the advantages of ethosomes and transferosomes to improve epidermal penetration and their high ethanol content (30%) set them apart¹⁹.

Transethasomal hydrogel preparations create novel drug delivery methods by combining the special benefits of hydrogels (hydrophilic polymers), ethosomes (lipid vesicles), and transferosomes. These formulations are very helpful for transdermal drug delivery, wound care, and dermatology. The main advantages of transethosomal hydrogel formulations, backed by research, will be discussed below.
Improved Bioavailability and Drug Penetration- It is well established that ethosomes, the active components of transethosomal formulations, enhance medication absorption into the skin and other biological membranes. They work by dissolving the lipid bilayer of the stratum corneum, increasing the skin's permeability and facilitating the deeper penetration of active pharmaceutical ingredients (APIs) into target tissues. Combining ethosomes and hydrogels greatly increases drug bioavailability. Hydrogels provide a moist environment that enhances skin penetration, while ethosomes allow the deeper transfer of active ingredients into the skin's layers.
Transferosomes- Transferosomes are more malleable liposomes that can better penetrate biological barriers such as the skin than ordinary liposomes. These innovative vesicles have revolutionized the drug delivery and cosmetics sectors. Transferosomes have shown great promise in improving skin penetration, removing biological barriers, and raising the bioavailability of active ingredients since their introduction in the 1990s. Key Features:
Ultra-deformable structure
High skin penetration ability
Enhanced bioavailability
Targeted delivery
Advantages
Improved efficacy
Decreased side effects
Better compliance by patient
Improved bioavailability
Transferosomes have unveiled new possibilities for active ingredient delivery and are promising due to their distinct characteristics for diverse applications. The benefits of ethosomes and transferosomes are combined in a new type of vesicular carriers called transethosomes. They are designed to enhance the transdermal delivery of medications, particularly those that struggle with skin permeability. Transethosomes have gained a lot of popularity recently due to their ability to improve the bioavailability and effectiveness of topical therapies. These ultra-deformable phospholipid vesicles' remarkable deformability and skin penetration properties, which allow for efficient drug delivery, are attributed to the presence of ethanol and water.²⁰
Main Features:
Ultra-deformable vesicle: Facilitates facile penetration through the skin.
High entrapment efficacy: Allows for effective drug loading.
Enhanced skin permeability: Increases drug delivery across the skin.
Biocompatibility: Made of natural phospholipids and ethanol.
Benefits:
Facilitates enhanced transdermal drug delivery.
Enhanced bioavailability and therapeutic efficacy of local drugs.
Minimised systemic adverse effects.
Better compliance of the patient.
Applications
Local delivery of analgesics, anti-inflammatory drugs, and antibiotics.
Therapy of skin diseases, like psoriasis and eczema.
Cosmetic use, for example, hydration of the skin and anti-aging²¹.
Controlled and Sustained Release of Active Ingredients - It is possible to release drugs that have been trapped in hydrogels gradually and carefully. When combined with ethosomes, this release can be further regulated, producing a longer-lasting therapeutic effect. This is particularly important for medications that must be taken consistently to address chronic diseases or discomfort.²²
Enhanced Skin Compatibility and Comfort - In dermatological applications where skin irritation can be a concern, hydrogels' biocompatibility and non-irritating properties, which often result in a soothing and moisturising effect, make them ideal for sensitive skin. Using ethosomal vesicles can improve the formulation's comfort and efficacy while lowering the risk of skin irritation associated with traditional delivery systems and guaranteeing improved active ingredient delivery²³.
Because hydrogels are biocompatible, non-irritating, and often have a soothing, moisturising effect, they are ideal for delicate skin. This characteristic is particularly helpful in dermatological applications where skin irritation may be a problem. By reducing the possibility of skin irritation linked to traditional administration methods and guaranteeing increased distribution of the active ingredient, ethosomal vesicles can improve the formulation's comfort and efficacy.
Transethosomal Hydrogel: Formulation & Benefits
Composition
Transethosomes (TEs):
Phospholipids: Form the vesicular bilayer, aiding in drug encapsulation and ensuring compatibility with the skin.
Ethanol: Increases the fluidity of vesicles and enhances skin permeability.
Edge Activators: Surfactants such as Tween 80 or Span 80 give vesicles more flexibility, which enhances their capacity to pass through the epidermal barrier.²⁴
Active Pharmaceutical Ingredient (API): The key ingredient that is intended for delivery.
Water: Serves as the medium for hydration.
Hydrogel Base:
Gelling Agents: Ingredients like Carbopol 934 or Hydroxypropyl Methylcellulose (HPMC) that create the gel structure.
pH Adjusters: Triethanolamine is one of the substances used to adjust the gel's pH so that it stays within an appropriate range for skin application.
Preservatives: Compounds added to prevent microbial growth, thereby extending the product's shelf life.²⁵
Preparation Steps
Preparation of Transethosomes:
Dissolve phospholipids, ethanol, and edge activators in water.
Utilize sonication or homogenization methods to develop ultra-flexible vesicles.
Add the drug to these vesicles.
Incorporation into Hydrogel:
Gradually mix the chosen gelling agent into water until fully dispersed.
Slowly incorporate the transethosomal suspension while stirring continuously to ensure uniform distribution.
Modify the pH and viscosity to reach the desired consistency suitable for topical application.
Benefits of Transethosomal Hydrogel
Increased Skin Permeation
Ultra-Deformable Vesicles: Transethosomes are made extremely flexible by the application of ethanol and edge activators, which improves their ability to pierce the stratum corneum.
Synergistic Effect: Edge activators lessen vesicle stiffness and ethanol breaks down the skin's lipid structure, which together enable deeper drug distribution.²⁶
Improved Drug Stability
Protective Environment: Encapsulation within transethosomes shields the drug from environmental degradation.
Hydrogel Matrix: The gel base stabilises the vesicles, preventing aggregation and maintaining the formulation's integrity over time
Sustained and Controlled Drug Release
Reduced Dosing Frequency: Sustained release patterns can result in less frequent application, improving patient compliance.
Hydrogel Network: The three-dimensional structure of the hydrogel permits a controlled release of the drug, sustaining therapeutic levels over extended periods of time.
Biocompatibility and Safety
Non-Irritating Components: Biocompatible materials are commonly used in formulations to reduce the possibility of skin irritation.
Improved Patient Compliance: Patients benefit from transethosomal hydrogels due to their non-invasive nature and lower side effect profile.²⁷
Versatility in Drug Delivery
Broad Drug Compatibility: Able to provide a variety of medicinal substances, such as hydrophilic and lipophilic medications.
Numerous Uses: Good for a range of ailments, including inflammation, fungal infections, and other skin-related diseases.
Transethosomal Hydrogel: Advantages over Conventional Systems
In contrast to traditional topical formulations such as creams, ointments, and normal hydrogels, transethosomal hydrogel is a sophisticated drug delivery method that combines transethosomes (ultra flexible lipid vesicles) with a hydrogel basis, enhancing drug penetration, stability, and therapeutic efficacy.
Enhanced Skin Permeation & Deeper Drug Delivery
High Deformability: Transethosomes are ultra-flexible vesicles that easily penetrate the stratum corneum (outermost skin layer), unlike conventional liposomes.
Ethanol & Edge Activators: Ethanol disrupts skin lipids, while surfactants increase vesicle elasticity, facilitating deep dermal and transdermal penetration.
Higher Drug Absorption: Provides better bioavailability than conventional hydrogels or creams.²⁶
Improved Drug Stability
Protective Vesicular Structure: Encapsulation within transethosomes prevents drug degradation (especially for sensitive drugs like peptides or antioxidants)
Hydrogel Network: The hydrogel matrix prevents vesicle aggregation, maintaining stability over time.
Longer Shelf Life: Less prone to oxidation and degradation compared to standard emulsions or gels.²⁷
Controlled & Sustained Drug Release
Hydrogel Matrix: Allows for gradual drug release, reducing frequent reapplication.
Prevents Rapid Clearance: Unlike creams, which are easily wiped off, transethosomal hydrogels adhere longer to the skin.
Reduces Side Effects: Sustained release minimises drug toxicity and irritation.²⁹
Non-Greasy & Patient-Friendly
Fast Absorbing & Non-Oily: Unlike ointments, transethosomal hydrogel is lightweight, non-sticky, and non-greasy.
Better Patient Compliance: More comfortable for long-term use in dermatological treatments.³⁰
Versatile & Suitable for Various Drugs
Delivers Both Hydrophilic & Lipophilic Drugs:
Hydrophilic drugs reside in the aqueous core of transethosomes.
Lipophilic drugs embed in the lipid bilayer.
Wide Applications: Used for psoriasis, acne, fungal infections, anti-inflammatory treatments, and pain management.³¹
Transethosomal Hydrogel: Characterization Techniques
Characterizing transethosomal hydrogels is essential to ensure stability, drug encapsulation, permeability, and efficacy. Various physicochemical, structural, and functional techniques are used to evaluate their properties.
Characterization of Transethosomes
Particle Size, Polydispersity Index (PDI), and Zeta Potential
Technique: Dynamic Light Scattering (DLS)
Purpose:
Determines size distribution (optimal size: 50–300 nm for deep skin penetration).
PDI (< 0.3 preferred) indicates homogeneity.
Zeta potential measures surface charge and colloidal stability (values ±30 mV suggest good stability).²⁶
Morphology & Vesicle Shape
Techniques: Transmission Electron Microscopy (TEM), Scanning Electron Microscopy (SEM), Atomic Force Microscopy (AFM)
Purpose:
TEM & SEM: Provide detailed visualization of vesicle structure, size, and shape.
AFM: Offers 3D surface imaging to confirm vesicle deformability and elasticity.²⁷
Encapsulation Efficiency (EE%) & Drug Loading (DL%)
Technique: UV-Vis Spectroscopy, High-Performance Liquid Chromatography (HPLC)
Purpose:
Measures EE% (percentage of drug successfully loaded inside vesicles).
HPLC ensures accurate quantification of the encapsulated drug.
Higher EE% (>70%) ensures better therapeutic efficiency.²⁸
Characterization of Hydrogel Base
Rheological Properties (Viscosity & Flow Behaviour)
Technique: Rotational Rheometer or Viscometer
Purpose:
Ensures optimal viscosity for spreading, adherence, and controlled release.
Confirms shear-thinning behaviour (gel becomes fluid upon application).
pH Measurement
Technique: pH Meter
Purpose: Maintains skin-friendly pH (5.5–7.4) to avoid irritation.²⁹
Drug Release & Kinetics
Technique: Franz Diffusion Cell
Purpose: Measures drug diffusion across synthetic membranes or human skin.
Confirms sustained release profile compared to conventional gels.²⁷
In Vitro & Ex Vivo Skin Permeation Studies
Technique: Franz Diffusion Cell with Human or Animal Skin
Purpose: Evaluates drug penetration depth in skin layers.
Confirms enhanced bioavailability of transethosomal hydrogel.²⁶
Clinical Studies
The promising results from preclinical studies underscore the potential of such formulations, but clinical trials are necessary to confirm their safety and efficacy in humans.
Comparison of Transethosomal Hydrogel with Existing Formulations
Compared to ordinary gels, ethosomes, liposomes, nanoemulsions, and other sophisticated drug delivery systems, transethosomal hydrogels have a number of advantages. A comparison based on important factors pertaining to topical and transdermal medication delivery may be seen below.
Skin Penetration Efficiency²⁶.
Formulation | Skin Penetration | Mechanism |
Liposomes | Low | Limited by rigid bilayer structure; mostly stays on the epidermis. |
Nanoemulsions | Moderate | Small droplet size enhances penetration but lacks active targeting. |
Ethosomes | High | Ethanol disrupts skin lipids, increasing drug permeation. |
Transferosomes | Very High | Ultra-deformable vesicles squeeze through skin pores. |
Transethosomal Hydrogel | Very High | Combines ethosomal skin penetration with transferosomal deformability, further enhanced by hydrogel retention. |
Drug Encapsulation & Stability²⁷
Formulation | Encapsulation Efficiency | Stability |
Liposomes | Moderate (40–70%) | Low; prone to aggregation and leakage. |
Nanoemulsions | Moderate to High (50–80%) | Stable but affected by phase separation. |
Ethosomes | High (60–85%) | Moderate; ethanol can cause vesicle instability. |
Transferosomes | High (70–90%) | Moderate; risk of vesicle fusion. |
Transethosomal Hydrogel | Very High (85–95%) | Excellent stability due to hydrogel matrix preventing aggregation. |
Drug Release Profile²⁷
Formulation | Release Type | Duration |
Liposomes | Burst Release | 6–12 hours |
Nanoemulsions | Sustained Release | 12–24 hours |
Ethosomes | Fast Penetration | 6–18 hours |
Transferosomes | Controlled Release | 18–24 hours |
Transethosomal Hydrogel | Sustained & Controlled Release | 24–48 hours due to hydrogel matrix. |
Suitability for Psoriasis Treatment²⁶
Formulation | Anti-Psoriatic Effectiveness | Retention on Skin |
Liposomes | Moderate | Low (washes off easily). |
Nanoemulsions | Moderate to High | Moderate (depends on viscosity). |
Ethosomes | High | Moderate. |
Transferosomes | Very High | Moderate to High. |
Transethosomal Hydrogel | Excellent | Very High (Hydrogel prolongs skin contact and drug action). |
Overall Advantages of Transethosomal Hydrogel
Superior Skin Penetration (Ethosomal and transferosomal synergy).
High Drug Encapsulation & Stability (Hydrogel prevents aggregation).
Sustained & Controlled Drug Release (Ideal for psoriasis management).
Better Retention & Patient Compliance (Non-greasy, long-lasting).
The TNF-α/IL-23/IL-17 axis is one of the immunological pathways that is dysregulated in psoriasis, a chronic inflammatory skin disorder. This route, which maintains inflammation and encourages keratinocyte proliferation, is essential to the pathophysiology of psoriasis. The development of successful medicines has focused on addressing these cytokines.
Challenges & Future Prospects
Challenges in formulation (stability, scale-up production, patient compliance).
Despite the promising potential of transethosomal hydrogels in psoriasis treatment, several challenges exist in their formulation, stability, scale-up production, and patient compliance
Stability Challenges²⁷
Issue | Impact | Possible Solutions |
Vesicle Aggregation & Fusion | Reduces drug bioavailability and efficacy. | Optimise surfactant and phospholipid ratio; use stabilizers (e.g., cholesterol, Tween-80). |
Ethanol-Induced Vesicle Leakage | Ethanol in ethosomes can destabilise vesicles over time, leading to premature drug release. | Optimise ethanol concentration (20–40% is optimal). |
pH Sensitivity | pH variations may alter hydrogel consistency and drug release. | Incorporate pH-buffering agents. |
Scale-Up & Production Challenges²⁷
Challenge | Impact | Potential Solutions |
Reproducibility Issues | Lab-scale formulations may not be reproducible in large batches. | Standardise preparation methods, ensure batch-to-batch consistency. |
High Production Costs | Specialised equipment and materials (e.g., phospholipids, ethanol) increase costs. | Optimise cost-effective excipients, explore alternative manufacturing techniques. |
Sterility & Contamination Risks | Hydrogels have high water content, increasing microbial growth risk. | Use preservatives, maintain aseptic processing conditions. |
Scalability of Vesicle Production | High-pressure homogenisation or probe sonication may be challenging to scale. | Develop alternative large-scale vesicle production methods such as microfluidics. |
Patient Compliance Challenges³²
Issue | Impact | Solution |
Skin Irritation from Ethanol | Ethanol may cause dryness or irritation in some patients. | Adjust ethanol concentration, add humectants (e.g., glycerine). |
Hydrogel Texture & Spreadability | If too sticky or greasy, patients may not adhere to the treatment. | Optimise viscosity and sensory properties. |
Dosage Accuracy | Patients may apply too much or too little. | Use metered-dose packaging for consistency. |
Long-Term Adherence | Requires sustained use for psoriasis management. | Improve user experience with non-greasy, fast-absorbing formulations. |
Regulatory Aspects of Transethosomal Drug Delivery Systems
Because transethosomes are advanced nanocarrier-based formulations meant for topical or transdermal use, they are subject to heightened scrutiny regarding safety, stability, manufacturing practices, and clinical effectiveness before receiving approval. Regulatory agencies such as the European Medicines Agency (EMA), the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), and the U.S. Food and Drug Administration (FDA) have established a number of guidelines that must be followed in order to obtain regulatory approval for transethosomal drug delivery systems.
Classification & Regulatory Pathway
The regulatory classification of transethosomal formulations depends on composition, intended use, and therapeutic claims:
Category | Regulatory Pathway | Example |
Transdermal Drug (NDA/ANDA) | Requires systemic bioavailability studies for approval. | Transdermal fentanyl patch. |
Cosmetic Product | Less stringent but must comply with safety and labelling regulations. | Moisturizers with transethosomal vitamin C. |
Nanomedicine or Complex Drug | Requires special regulatory consideration for nanoparticle-based formulations. | Liposomal doxorubicin (Doxil). |
Key Regulatory Challenges³³
Challenge | Regulatory Concern | Requirements |
Nanocarrier Safety | Possible toxicity, bioaccumulation, and systemic absorption. | Toxicity studies (in vitro & in vivo), skin irritation tests. |
Stability & Shelf-Life | Ethanol in ethosomes may cause instability & vesicle fusion over time. | Long-term & accelerated stability studies (ICH Q1A guidelines). |
Manufacturing Consistency | Nano-size vesicles require strict batch-to-batch uniformity. | Good Manufacturing Practices (GMP), Quality by Design (QbD). |
Drug Release & Skin Penetration | Unpredictable drug release due to ethosomal and transferosomal interactions. | In vitro drug release tests, Franz diffusion cell studies, ex vivo permeation studies. |
Clinical Efficacy | Need to prove therapeutic superiority over conventional formulations. | Phase I–III clinical trials required. |
Regulatory Requirements for Transethosomal Hydrogel Approval
Preclinical Studies (Non-Clinical Studies)
Toxicity Assessment (Acute, Sub-chronic, Chronic)
Skin Irritation, Sensitization, and Allergic Potential
Pharmacokinetics (Absorption, Distribution, Metabolism, Excretion - ADME)
In Vitro & Ex Vivo Skin Permeation Studies³⁴
Clinical Studies (Human Trials - FDA IND Application)
Phase I: Safety & Tolerability (small sample)
Phase II: Efficacy & Dose Optimisation
Phase III: Large-Scale Clinical Trial (Comparative with standard treatments)
Phase IV: Post-Marketing Surveillance (Pharmacovigilance)³⁵.
Good Manufacturing Practices (GMP) & Quality Control
Compliance with GMP and ICH guidelines is mandatory for production scale-up:
Sterility & Microbial Control (Water-based hydrogels prone to contamination).
Nano-vesicle Characterization (Size, Zeta Potential, Entrapment Efficiency).
Product Stability Studies (Temperature, Humidity, Light Exposure).
Batch-to-Batch Consistency (Ensuring reproducibility in transethosomal vesicle formation)³⁶].
Market Approval & Post-Market Surveillance (Pharmacovigilance)
It is essential to maintain safety monitoring through Phase IV trials, pharmacovigilance databases, and adverse event reporting after a transethosomal medication product is approved.
Examples of Marketed Nanocarrier Drugs: • Liposomal Amphotericin B (Ambisome) - Antifungal • Ethosomal Tacrolimus Gel - Experimental for Psoriasis³⁷.
Future research directions (nanocarriers, combinational therapy, clinical trials).
Advanced Nanocarrier Design & Functionalisation
To improve drug stability, penetration, and bioavailability, we should consider exploring next generation nanocarriers:
Hybrid Nanocarriers: Merging transethosomes with polymeric or lipid nanoparticles to boost stability. Stimuli-Responsive Hydrogels: Developing pH-sensitive or enzyme-triggered hydrogels for controlled drug release.
Smart Nanocarriers: Modifying the surfaces of ethosomes (through PEGylation or ligand conjugation) for targeted therapy.
Bioinspired Carriers: Utilizing exosomes or lipid-based nanovesicles to enhance skin penetration.
Example: Chitosan-coated transethosomes have shown better penetration and sustained release.
Large-Scale Clinical Trials
To validate transethosomal hydrogel safety & efficacy, well-designed clinical trials are needed:
Proposed Clinical Research Plan
Trial Phase | Objective | Key Parameters |
Phase I | Safety & Tolerability | Skin irritation, systemic absorption, adverse reactions. |
Phase II | Efficacy vs. Standard Treatments | PASI (Psoriasis Area Severity Index) reduction, cytokine inhibition (IL-17, TNF-α). |
Phase III | Long-Term Effectiveness | Relapse rate, patient compliance, quality of life. |
Phase IV | Post-Marketing Surveillance | Pharmacovigilance, rare side effects. |
Example: Clinical trials on ethosomal tacrolimus showed improved bioavailability over conventional gels.
Regulatory & Commercialization Strategies
To successfully bring transethosomal hydrogels to market, research should also concentrate on:
GMP-Scale Manufacturing: Creating cost-effective production methods to facilitate commercialisation.
Regulatory Approval Pathways: Accelerating approvals through orphan drug designations for severe psoriasis cases.
Patient-Centric Approaches: Developing non-greasy, fast-absorbing hydrogels to enhance patient compliance.
AI & Machine Learning: Leveraging AI-driven drug formulation modelling to refine nanocarrier properties.
Example: AI-driven transdermal formulation modelling has been used to optimise nanoemulsions for drug penetration³⁸.
Conclusion
The research highlights the potential of transethosomal hydrogels as an innovative nanocarrier system for treating psoriasis, addressing key limitations of conventional therapies such as poor drug penetration, instability, and difficulties in patient compliance. By integrating the benefits of ethosomes, transferosomes, and hydrogels, these formulations improve skin permeability, enhance drug stability, and enable controlled drug release, resulting in better therapeutic outcomes. The transethosomal hydrogel system allows for deeper drug penetration, longer retention, and fewer systemic side effects, making it a promising option for dermatological treatments. However, challenges persist in scaling up production, optimizing stability, and obtaining regulatory approvals. Future studies should prioritize clinical validation, ensure long-term safety, and investigate new functionalised nanocarriers for targeted therapies. Additionally, advancements in combination therapies and AI-driven formulation techniques could further enhance effectiveness. With continued research and regulatory support, transethosomal hydrogels hold great promise for revolutionizing psoriasis treatment and improving patients' quality of life.
References
1. Tsoi, L.C., Spain, S.L., Knight, J., Ellinghaus, E., Stuart, P.E., Capon, F., Ding, J., Li, Y., Tejasvi, T., Gudjonsson, J.E. and Kang, H.M., 2012. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nature genetics, 44(12), pp.1341-1348.
2. Bowcock, A.M. and Krueger, J.G., 2005. Getting under the skin: the immunogenetics of psoriasis. Nature Reviews Immunology, 5(9), pp.699-71.
3. Nickoloff, B.J. and Nestle, F.O., 2004. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. The Journal of clinical investigation, 113(12), pp.1664-1675.
4. Boyman, O., Hefti, H.P., Conrad, C., Nickoloff, B.J., Suter, M. and Nestle, F.O., 2004. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. The Journal of experimental medicine, 199(5), pp.731-736.
5. Reich, K., Nestle, F.O., Papp, K., Ortonne, J.P., Evans, R., Guzzo, C., Li, S., Dooley, L.T. and Griffiths, C.E., 2005. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. The Lancet, 366(9494), pp.1367-1374.
6. Harlow, D., Poyner, T., Finlay, A.Y. and Dykes, P.J., 2000. Impaired quality of life of adults with skin disease in primary care. British Journal of Dermatology, 143(5), pp.979-982.
7. Fortune, D.G., Richards, H.L. and Griffiths, C.E., 2005. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatologic clinics, 23(4), pp.681-694.
8. De Korte, J., Mombers, F.M., Bos, J.D. and Sprangers, M.A., 2004, March. Quality of life in patients with psoriasis: a systematic literature review. In Journal of Investigative Dermatology Symposium Proceedings (Vol. 9, No. 2, pp. 140-147). Elsevier.
9. Rapp, S.R., Feldman, S.R., Exum, M.L., Fleischer Jr, A.B. and Reboussin, D.M., 1999. Psoriasis causes as much disability as other major medical diseases. Journal of the American Academy of Dermatology, 41(3), pp.401-407.
10. Stern, R.S., Nijsten, T., Feldman, S.R., Margolis, D.J. and Rolstad, T., 2004, March. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. In Journal of Investigative Dermatology Symposium Proceedings (Vol. 9, No. 2, pp. 136-139). Elsevier.
11. Touitou, E., Dayan, N., Bergelson, L., Godin, B. and Eliaz, M., 2000. Ethosomes—novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. Journal of controlled release, 65(3), pp.403-418.
12. Cevc, G. and Vierl, U., 2010. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. Journal of controlled release, 141(3), pp.277-299.
13. Manosroi, A., Jantrawut, P., Khositsuntiwong, N., Manosroi, W. and Manosroi, J., 2009. Novel elastic nanovesicles for cosmeceutical and pharmaceutical applications. Chiang Mai J Sci, 36(2), pp.168-178.
14. Nandure, H.P., Puranik, P., Giram, P. and Lone, V., 2013. Ethosome: A Novel Drug Carrier. International journal of pharmaceutical research & allied sciences, 2(3).
15. Romero, E.L. and Morilla, M.J., 2013. Highly deformable and highly fluid vesicles as potential drug delivery systems: theoretical and practical considerations. International journal of nanomedicine, pp.3171-3186.
16. Vinod, K.R., Kumar, M.S., Anbazhagan, S., Sandhya, S., Saikumar, P., Rohit, R.T. and Banji, D., 2012. Critical issues related to transfersomes-novel vesicular system. ACTA ScientiarumPolonorumTechnologiaAlimentaria, 11(1), pp.67-82.
17. Cevc, G., Blume, G., Schätzlein, A., Gebauer, D. and Paul, A., 1996. The skin: a pathway for systemic treatment with patches and lipid-based agent carriers. Advanced drug delivery reviews, 18(3), pp.349-378.
18. Song, C.K., Balakrishnan, P., Shim, C.K., Chung, S.J., Chong, S. and Kim, D.D., 2012. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Colloids and surfaces B: biointerfaces, 92, pp.299-304.
19. Ahmed, E.M., Aggor, F.S., Awad, A.M. and El-Aref, A.T., 2013. An innovative method for preparation of nanometal hydroxide superabsorbent hydrogel. Carbohydrate polymers, 91(2), pp.693-698.
20. Sayed, A., et al. (2018). "Safety and irritation potential of topical agents in dermatological therapy." Clinical Dermatology, 36(5), 437-445.
21. Boffetta, P., Gridley, G. and Lindelöf, B., 2001. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. Journal of Investigative Dermatology, 117(6), pp.1531-1537.
22. "Mohite, P., Rahayu, P., Munde, S., Ade, N., Chidrawar, V.R., Singh, S., Jayeoye, T.J., Prajapati, B.G., Bhattacharya, S. and Patel, R.J., 2023. Chitosan-based hydrogel in the management of dermal infections: a review. Gels, 9(7), p.594.
23. Rapp, S.R., Feldman, S.R., Exum, M.L., Fleischer Jr, A.B. and Reboussin, D.M., 1999. Psoriasis causes as much disability as other major medical diseases. Journal of the American Academy of Dermatology, 41(3), pp.401-407.
24. Sun, S., Zhang, X., Xu, M., Zhang, F., Tian, F., Cui, J., Xia, Y., Liang, C., Zhou, S., Wei, H. and Zhao, H., 2019. Berberine downregulates CDC6 and inhibits proliferation via targeting JAK-STAT3 signaling in keratinocytes. Cell Death & Disease, 10(4), p.274.
25. Michalek, I.M., Loring, B. and John, S.M., 2017. A systematic review of worldwide epidemiology of psoriasis. Journal of the European Academy of Dermatology and Venereology, 31(2), pp.205-212.
26. Bin Jardan, Y.A., Ahad, A., Raish, M. and Al-Jenoobi, F.I., 2023. Preparation and characterization of transethosome formulation for the enhanced delivery of sinapic acid. Pharmaceutics, 15(10), p.2391.
27. Asghar, Z., Jamshaid, T., Sajid-ur-Rehman, M., Jamshaid, U. and Gad, H.A., 2023. Novel transethosomal gel containing miconazole nitrate; development, characterization, and enhanced antifungal activity. Pharmaceutics, 15(11), p.2537.
28. Bevinakoppamath, S.S., Dandagi, P.M., Hulyalkar, S. and Biradar, P., 2024. Formulation, Evaluation and Optimization of Acitretin Loaded Transethosomes for the Management of Psoriasis. Journal of Pharmaceutical Innovation, 19(5), p.51.
29. Abdulbaqi, I.M., Darwis, Y., Assi, R.A. and Khan, N.A.K., 2018. Transethosomal gels as carriers for the transdermal delivery of colchicine: statistical optimization, characterization, and ex vivo evaluation. Drug design, development and therapy, pp.795-813.
30. Ma, J., Wang, Y. and Lu, R., 2022. Mechanism and application of chitosan and its derivatives in promoting permeation in transdermal drug delivery systems: a review. Pharmaceuticals, 15(4), p.459.
31. Mezei, M. and Gulasekharam, V., 1980. Liposomes-a selective drug delivery system for the topical route of administration I. Lotion dosage form. Life sciences, 26(18), pp.1473-1477.
32. Mulani, H.; Bhise, K.S. QbD Approach in the formulation and evaluation of Miconazole Nitrate loaded ethosomal cream-o-gel. Int. Res. J. Pharm. Sci. 2017, 8, 1–13.
33. Niazi, S.K., Al-Shaqha, W.M. and Mirza, Z., 2023. Proposal of international council for harmonization (ICH) guideline for the approval of biosimilars. Journal of Market Access & Health Policy, 11(1), p.2147286.
34. Stoykova, K., 2025. Towards Non-Animal Testing in European Regulatory Toxicology: An Introduction to the REACH Framework and Challenges in Implementing the 3Rs. European Journal of Risk Regulation, pp.1-32.
35. Cedeno-Serna, J., 2023. Investigational new drug (IND) application. In Translational Sports Medicine (pp. 419-424). Academic Press.
36. Oltmanns, J., Macherey, M., Schwarz, M., Manžuch, Z., Hayleck, M. and Heine, K., 2023. Mapping of Data Requirements and Assessment Methodologies Linked to the Regulatory Frameworks and Remits of the Relevant EU Agencies (ECHA, EFSA and EMA) and EC Scientific Committees (SCCS and SCHEER) Final Report. EFSA Supporting Publications, 20(12), p.8540E.
37. Guo, M., Shu, Y., Chen, G., Li, J. and Li, F., 2022. A real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events for niraparib. Scientific reports, 12(1), p.20601.
38. Dwivedi, K., Regi, J.M., Cleveland, T.J., Turner, D., Kusumawidjaja, D., Thomas, S.M. and Goode, S.D., 2019. Confirmation of no common femoral artery stenosis following percutaneous EVAR. Cardiovascular and Interventional Radiology, 42, pp.1369-1370.
Author Information
Authors:
Jayesh Mutha, Sweta Srivastava Koka*, Sumeet Dwivedi, Ravi Sharma, Ashish Gupta, Pravin Kumar Sharma and G. N. Darwhekar
Acropolis Institute of Pharmaceutical Education and Research, Indore, Madhya Pradesh, India*
E-Mail address: sweta.koka@gmail.com
Corresponding Author:
Sweta Srivastava Koka*
Address: Acropolis Institute of Pharmaceutical Education and Research, Indore, Madhya Pradesh, India
Email: Email: sweta.koka@gmail.com




Comments