- tamsinmarshall4
- Jul 11, 2025
- 41 min read
Updated: Jul 14, 2025
Technical Review Article | Open Access | Published 11th July 2025
From Concept to Delivery: The Evolution and Applications of Hot-Melt Extrusion in Pharmaceuticals
Nensi Raytthatha, Krishna School of Pharmacy & Research | EJPPS | 302 (2025)| https://doi.org/10.37521/ejpps30209
Abstract
Hot Melt Extrusion (HME) is an innovative approach that is currently receiving significant interest in the pharmaceutical sector for the manufacturing of various dosage forms. This technique entails the melting and extruding of a mixture of pharmaceutical drugs and polymeric carriers, resulting in solid dispersions or more sophisticated formulations. This review examines the historical development of HME and its foundational applications in pharmaceutical manufacturing. It also provides an overview of the type of extruders used for hot melt extrusion and analyzes the essential extrusion parameters that affect the qualities of the product. Furthermore, it analyzes the variety of materials appropriate for HME, including both active pharmaceutical ingredients and polymeric carriers. HME is not only a validated manufacturing method but also aligns with the objectives of the US Food and Drug Administration's (FDA) process analytical technology (PAT) framework for the design, analysis, and control of production through quality control metrics throughout the active extrusion process. From a comprehensive viewpoint, hot-melt extrusion technology encompasses its components, processing technologies, materials and innovative formulation designs and advancements in many applications for oral drug delivery system.
Keywords: Screw extruders, manufacturing, solubility, bioavailability, extrusion, solubility enhancement
1. INTRODUCTION
The pharmaceutical industry continually faces the challenge of enhancing the solubility and bioavailability of poorly water-soluble drugs, which make up a significant portion of newly discovered therapeutic agents. Nearly 40% of new drug candidates are classified under the Biopharmaceutics Classification System (BCS) Class II and IV, characterized by low solubility and/or low permeability. This categorization reflects a growing need for innovative solubility enhancement techniques to optimise drug delivery. These techniques aim to modify the physicochemical properties of drugs, improving their solubility, dissolution rates, and overall bioavailability, thereby maximizing their therapeutic potential. Various approaches, including particle size reduction, solid dispersions, salt formation, and cocrystallization, are employed to address the solubility challenges in drug development¹.
Solubility enhancement techniques, which involve modifying the physical characteristics of drugs, have emerged as promising strategies. By altering factors such as particle size, crystallinity, and surface properties, these techniques can significantly improve drug dissolution and absorption. Common approaches include solid dispersions, cocrystals, salt formation, and lipid-based technologies. In addition to the above techniques, advanced manufacturing techniques play a pivotal role in producing efficient and scalable drug delivery systems.
Extrusion is one such manufacturing technique that has gained considerable attention in recent years. Two common extrusion methods are cold extrusion and hot melt extrusion. Cold extrusion involves the processing of materials at room temperature or slightly elevated temperatures without melting them. It is suitable for heat-sensitive drugs and materials that may degrade at higher temperatures. This technique relies on the mechanical force to push the material through a die to form a desired shape, often followed by drying or curing steps. In contrast, as shown in Table 1, Hot Melt Extrusion (HME) involves the use of heat to melt the polymer-drug mixture, which is then pushed through a die to form a solid dosage form upon cooling. HME offers several advantages, such as the ability to enhance drug solubility and bioavailability, the formation of solid dispersions, and the capability to process a wide range of polymers. However, it requires careful temperature control to prevent thermal degradation of heat-sensitive compounds. HME involves processing drug-polymer mixtures at elevated temperatures, enabling the formation of solid dispersions and other innovative formulations. HME is highly efficient in enhancing solubility as it converts drugs with low solubility into an amorphous state, hence significantly increasing their surface area and rate of dissolution. Heat and shear forces applied in hot melt extrusion (HME) help disperse the drug evenly throughout the polymer matrix, decreasing crystallinity and improving the drug's capacity to dissolve and be absorbed by the body. HME is a favourable option for developing pharmaceuticals with low solubility in water due to its ability to surpass the constraints of conventional techniques and offer a reliable, expandable, and uninterrupted manufacturing (continuous manufacturing). This review has a 3-fold aspiration:
Firstly, to explore the intersection of some solubility enhancement techniques and manufacturing methods, focusing on hot-melt extrusion.
Secondly, to elucidate the designed extrusion instrument parts together with their functions and to offer a comprehensive overview of the process of HME and the raw materials utilized therein.
The third objective is to showcase newly published papers on the utilization of the HME process in the advancement of diverse medication administration methods, including 3D printed dosage forms, solid dispersions, lipid-based methodologies, and taste masking, etc.².
HME emerged in plastic manufacturing companies during the second decade of the 20th century. Since that time, its application has significantly expanded ³. HME is becoming more utilized in processing methods within the industry. The rise in the acceptance of HME has led to the successful formulation of pharmaceuticals characterized by low solubility, low bioavailability, and high potency or dosage for diverse applications, including targeted drug release, modified drug release, abuse-deterrent, and taste-masking. HME has emerged as a viable alternative to traditional methods for manufacturing various topicals, oral, and injectable formulations due to its potential benefits. Recent advancements in HME have enabled the treatment of temperature-sensitive materials and thermally unstable substances, including temperature-sensitive drugs, amino acids, and proteins, resulting in improved stability ⁵⁻⁷. Pharmaceutical industries continue to encounter various challenges in the development of drug delivery systems, including those related to low therapeutic effectiveness, residual solvents, and the presence of bitter-tasting drugs. HME technology effectively resolves these problems.
HME technology has several essential benefits, such as uninterrupted production, cost-effectiveness, absence of solvents, practicality for industrial use, possibility for automation, high level of reproducibility, and the ability to monitor in real-time. However, obstacles such as elevated operating temperature, shear rates, high energy input, and the lack of excipients that comply with HME standards may have hindered the wider use of this technique ³. Although the constraints exist, these challenges can overcome by extensive research conducted over time by implementing innovative methodologies and adjusting equipment. Additionally, the use of suitable additives and the availability of a wide range of extrudable excipients for HME contribute to addressing these concerns. In modern times, HME can be combined with additional downstream auxiliary process, such as a 3D printing, a high-pressure homogenization, or a pelletization to address patient concerns.
Parameter | Cold Extrusion (CE) | Hot Melt Extrusion (HME) |
Temperature | Low or ambient temperatures (room or slightly elevated) | High temperatures to melt the polymer and drug (above melting point of polymer/carrier) |
Suitability | Suitable for thermolabile substances and drugs | Not suitable for thermolabile substances and drugs. |
Drug-polymer interaction | Minimal interaction due to low temperatures. | Enhanced interaction due to molten state, promoting better dispersions. |
Drug Form | Typically maintains crystalline drug form | Converts drug into an amorphous form |
Thermal Degradation | Minimal risk due to low temperature | Risk of degradation if not controlled |
Mixing | Limited mixing capability | Excellent mixing and dispersion capabilities |
Equipment | Simple equipment, lower energy consumption | Requires specialized equipment, higher energy consumption |
Product Form | Semisolid or solid forms | Solid dispersions, films, granules |
Process Complexity | Simple | More complex, requiring control of temperature and shear |
Process scalability | Less scalable, batch process | Highly scalable, continuous process |
Cost and efficiency | Lower cost, less efficiency for solubility enhancement but has efficiency for thermolabile drugs and substances. | Higher cost but more efficient in enhancing solubility |
Diagrammatic representation |  |  |
Historical developments of Hot Melt Extrusion
Test organisms and granulate inoculation
In the year 1930, the HME was initially introduced in the polymer industry for processing plastics and rubber. However, its potential in pharmaceuticals was realized in the 1950s. The pharmaceutical industry began exploring HME for continuous processing, particularly for sustained-release formulations. In the 1970s, researchers observed solubility and bioavailability enhancement potential of poorly water-soluble drugs through solid dispersions. In the 1980s, HME was used to develop controlled-release dosage forms, utilizing polymers like Ethylcellulose to control drug release rates ⁴.
Finally, in the 1990s, the first FDA-approved pharmaceuticals manufactured using HME technology were introduced. The method became a tool for producing solid dispersions, improving drug delivery for poorly soluble drugs. Research focused on polymeric carriers like polyethylene glycol (PEG) and polyvinylpyrrolidone (PVP), leading to enhanced drug dissolution and stability. In the 2000s, HME gained mainstream adoption in the pharmaceutical industry, becoming a preferred method for improving the bioavailability of Biopharmaceutics Classification System (BCS) class II drugs. Advancements in screw designs and temperature control allowed for the extrusion of thermolabile drugs.
In the 2010s, HME was integrated with 3D printing and advanced drug delivery systems, opening new possibilities for personalized medicine. Researchers began using HME to extrude filaments for 3D printing of patient-specific dosage forms.
Complex Drug Delivery Systems: HME began to be integrated with other innovative drug delivery technologies, such as nanoparticles, microparticles, and solid dispersions. In the 2020s, the FDA promoted continuous manufacturing, promoting HME as a preferred technology for producing uniform, high-quality drug products. Lipid-based technologies and extrusion were advanced to address specific drug delivery challenges. Research into sustainable polymers was initiated to reduce the environmental impact of pharmaceutical manufacturing. The detailed history of hot melt extrusion is as shown in Figure 1.⁵

Hot Melt Extrusion Manufacturing
Hot melt extrusion (HME) is a manufacturing method where a substance is heated at high temperatures until it melts or becomes soft. It is driven into a mould, often using one or two conveyor screws in a cylindrical container. The process may be separated into numerous stages, which includes heating the material, feeding, mixing, and conveying, allowing it to pass through the dye, and processing the material downstream. Each of these phases may be regulated and have an impact on the ultimate characteristics of the product.
Commercial extruders are sophisticated machines utilized in the manufacturing of a diverse range of products. These systems typically consist of a feeder, an extrusion die, and a conveyor barrel designed to shape the material as it passes through the extruder. The barrel is often secured using clamps to ensure stability during operation. Additional components may include heating or cooling systems, degassing units, conveyor mechanisms such as caterpillar belts, and solvent supply pumps. Advanced configurations may incorporate inline lasers to measure strand diameters with precision. Extruders are often integrated with downstream processing equipment, enabling the production of either final dosage forms or intermediate materials for subsequent processing. Co-extrusion techniques are commonly applied, allowing distinct materials to flow through separate channels and converge at a shared die, producing multilayered structures with tailored properties ⁶.
Pharmaceutical screw extruders are designed to meet regulatory requirements for dosage form production. They operate at a specific screw rotation speed, considering torque and shear rate generated by the material and screw design. The extrusion assembly includes an electric motor, extrusion barrel, rotating screw, and endplate die, determining the form of the product. The screw flights and barrel wall guide the material within the extruder. A central electronic control system regulates process parameters such as screw speed, feed rate, temperature, and vacuum levels. These extruders are categorized into single-screw extruders (SSEs), twin-screw extruders (TSEs), and multi-screw extruders (MSEs), each designed for specific applications and operational efficiencies ⁷ as discussed in Table 2.
Single screw extruders (SSEs)
SSEs are commonly used extruders owing to their mechanical simplicity and minor alterations since their inception in 1897 ⁸. Usually, SSEs comprises of a continuously revolving screw within a barrel, yielding high-quality molten material and maintaining stable pressure for uniform output. The screw design has twenty or more helical turns, resulting in an elongated apparatus with significant temperature differentials and ample residence time for thorough mixing from one end to the other. SSEs can execute many processes, including the feeding of ingredients, conveying, melting, devolatilizing, pumping, & shaping. The screw flights and the inner surface of the barrel together create a flow channel, facilitating the transport of materials toward the proximal section of the screw in a single-screw extruder (SSE). The elevated temperature of the barrel surface, combined with the mechanical energy imparted by the screw, lead to the formation of a molten pool that progressively enlarges as the solid bed diminishes in size. The resulting molten material is then directed through a die for subsequent processing ⁹.
Twin screw extruders (TSEs)
The first TSE was introduced in Italy during the late 1930s, that amalgamated the mechanical functions of multiple apparatus into a single apparatus. It comprises of two agitator assemblies affixed to parallel shafts, facilitating various configurations and conditions across all extruder zones, from input feeding to conveying to the regulated pumping ¹⁰. TSEs can be co-rotating or counter-rotating and can be grouped as fully intermeshing or non-intermeshing. The fully intermeshing TSE is the best choice of screw because of its self-cleaning capabilities, which reduces non-motion and localised overheating of raw materials. Non-intermeshing TSEs are less desirable in mixing applications because of reduced screw interactions and poor self-cleaning efficiency. Non- intermeshing TSEs are frequently employed for the processing of very viscous materials and for the extraction of large volumes of volatile chemicals ⁹.
Multi screw extruders (MSE)
MSE extruders have more than two screws, with their configurations differing based on the quantity of screws employed and based on their use in the pharmaceutical industry. For instance, three or five screws are positioned linearly whereas, six or eight screws are arranged circumferentially. Multi-screw extruders (MSEs) are preferred over single-screw extruders (SSEs) due to the upward displacement flow occurring in the intermeshing regions between the screws, which effectively minimises thermal degradation of heat-sensitive materials. This advantage arises from the shear-dominated flow dynamics of the molten material within the MSE, ensuring more controlled processing conditions ¹¹.
Table 2: Difference between extruders
Parameters | Single screw extruder | Twin screw extruder | Multi screw extruder |
Design & Configuration | Contains one rotating screw in a barrel. | Contains two co-rotating or counter-rotating screws in the barrel. | Contains more than two screws, usually 3 or more in parallel. |
Processing Capability | Best for simple, continuous processes (e.g., simple mixing, melting). | Manages more complex processes (e.g., mixing, compounding, devolatilization, etc.). | Manages highly complex processes (e.g., high shear mixing, high viscosity materials). |
Mixing Efficiency | Limited mixing capability due to low shear and no intermeshing. | High mixing efficiency due to intermeshing screws and better shear control. | Highest mixing efficiency with enhanced shear and compression forces. |
Shear Stress | Generates low shear forces. | Produces higher and controllable shear forces, especially in co-rotating setups. | Generates maximum shear forces, allowing precise control of material properties. |
Temperature Control | Basic temperature control, often limited to barrel heating. | Advanced temperature control due to screw configurations that allow zonal heating and cooling. | Most advanced temperature control with more precise zonal management. |
Cost & Maintenance | Lower cost; simpler to maintain due to fewer components. | Higher cost due to complex design but offers better performance and flexibility. | Highest cost and most complex maintenance, often used for specialized tasks. |
Screw Speed | Lower screw speed, usually slower processing. | High-speed operation with flexibility in adjusting screw speed. | Capable of operating at the highest speeds, with precise control of processing conditions. |
Residence Time of Material | Longer residence time, which may affect heat-sensitive materials. | Shorter, more controlled residence time, suitable for heat-sensitive drugs. | Very short, tightly controlled residence time, ideal for sensitive and complex materials. |
Energy Consumption | Lower energy consumption due to simpler design. | Higher energy consumption due to dual screws and complex functions. | Highest energy consumption due to multiple screws and advanced functionalities. |
Scalability | Scalable for smaller, simpler production lines. | Highly scalable for both medium and large-scale production. | Best suited for large-scale, continuous manufacturing processes. |
Diagram |  |  |  |
Extrusion process parameters
Temperature
The temperature is a critical factor in the process of extrusion as the polymer needs to be heated above its Tg (glass transition temperature) while remaining below its Tdeg (degradation temperature). The API can be evaluated in relation to its Tm (Melting temperature), depending on whether a miscibility or solubility parameter is used¹². Breitenbach noted that the viscosity of the molten mass is strongly temperature dependent. A balance must be achieved between lower temperatures, which result in higher viscosity and elevated torque, and higher temperatures, which reduce torque due to decreased viscosity but risk degradation of the polymer and active pharmaceutical ingredient (API). Mechanical energy is typically transferred to the molten mass through the action of the screws ¹³. Nikitine et al. demonstrated that temperature has a profound impact on flow rate, as the viscosity of a polymer is intrinsically linked to its flow characteristics. Their study revealed that increasing the barrel temperature improves the filling rate while concurrently reducing the residence time of the product within the extruder. ¹⁴. Jijun et al. developed amorphous extrudates containing 15% nimodipine and Kollidon VA64. Kollidon1 VA64 was found to be excessively viscous at temperatures below 130°C, which resulted in the retention of crystalline nimodipine. Nonetheless, temperatures exceeding 160°C resulted in the extrudates becoming brown, signifying the degradation of nimodipine ¹⁵. The authors determined that to achieve extrudates with amorphous nimodipine without degradation, barrel temperature must be maintained below 150°C, and the viscosity of Kollidon1 VA64 should be appropriate. Almeida et al. prepared extrudates containing 50% metoprolol tartrate (MPT) ethylene vinyl acetate at different temperatures. It was observed that as the temperature rises, the percentage of crystallinity of metoprolol tartrate decreases, a finding that was estimated through differential scanning calorimetry¹⁶.
The barrel temperature during extrusion influences the final product, as well as parameters such as residence time and melt viscosity. The optimal temperature for extrusion for each polymer/API (active pharmaceutical ingredient) combination must be established. Operating at low temperatures decreases mixing capacity and increases extruder torque, resulting in substandard cocrystals and amorphous dispersions. The maximum operating temperature must be established to prevent polymer or API degradation. An equilibrium must be established between low temperatures resulting in poor quality of final products and high temperatures leading to compound degradation.
Screw configuration/type/size.
The screw arrangement can be modified to change the manufacturing technique. By optimizing the individual screw parts, it is possible to tailor them to specific purposes ¹³⋅¹⁷. Furthermore, the amount of time the mixture remains inside the barrel will also be affected by the element type utilized in the procedure. For instance, kneading properties will extend the time of residence.
Nakamichi et al. established that the incorporation of a mixing zone is essential for achieving homogeneous and consistent extrudates in the preparation of nifedipine with hydroxypropyl methylcellulose phthalate. It was determined that positioning the shaping paddle at the two-thirds mark of the barrels is optimum for this purpose. The samples are immediately obtained from the barrel and screw and analyzed using DSC (Differential Scanning Calorimetry) and XRD (X-ray Diffraction). Moreover, during the examination of drug release in a regulated laboratory setting, supersaturation was seen solely in the presence of the kneading paddle ¹⁸. Verhoeven et al. discovered a similar outcome when extruding ethylcellulose (EC) with metoprolol tartrate. Study findings suggested by Verhoeven et al, indicated that while the quantity and placement of mixing zones within the barrel can be modified, these alterations do not affect the effectiveness of the combination or the drug release ¹⁹.
Lang et al. proposed an innovative approach by altering the size of the screw rather than the screw configuration, resulting in a unique type of extruder. The study utilized polymers such as hydroxypropyl methylcellulose acetate succinate (HPMCAS), polyethylene oxide (PEO), and Polyox to process the interfacial transition zone (ITZ). Three distinct extruder types were employed: a single-screw extruder, a small-scale twin-screw extruder featuring conical twin screws, and a 16 mm twin-screw extruder equipped with kneading elements. The primary variable under investigation was the mixing capacity, which ranged from low to medium and high. The findings highlighted that only the 16 mm twin-screw extruder demonstrated the capability to produce solid dispersions effectively ²⁰.
In a study conducted by Gosh et al., the processing of thermally sensitive drug compounds revealed the critical influence of kneading block positioning within a twin-screw extruder. The researchers observed that placing the kneading block near the feed section led to the premature melting of the API. Consequently, the API remained in the extrusion barrel for a prolonged duration, increasing its susceptibility to thermal degradation and compromising the stability of the compound ²¹.
Nevertheless, when the kneading block was placed at the end of the barrel, there was a noticeable delay in the melting of the API, which resulted in enhanced stability of the API. Ultimately, the kind, configuration, and design of the screw do impact the many aspects associated with the extrusion process. Typically, a combination of high mixing capacity and a screw arrangement with strong shear has been proven to yield the most favorable outcomes. When it comes to amorphization and cocrystallization, the twin screw extruder is the preferable approach over single screw extruder due to its superior mixing capability. Furthermore, placing a kneading zone either two-thirds of the way or at the end of the barrel is often enough to promote thorough mixing at the molecular level and provide superior cocrystals and solid dispersions. However, it is important to note that each kneading zone contributes to a longer residence time. If there are an excessive number of these zones, there is a risk of degradation of the polymer or API owing to the shear forces and prolonged residence time. This risk is particularly significant when the paddle angle exceeds 30 degrees.
Screw speed
In extrusion procedures, the results of material processing are greatly affected by the screw speed, as pointed out by Reitz et al. Their investigation used twin-screw hot melt extrusion with mannitol and griseofulvin and revealed that the revolution speed of the screw has a substantial impact on the load inside the barrel, the mixing efficiency, and the shear rate of the process. Particle size was also found to be controlled by differences in screw speed. Also, inadequate loading at lower screw speeds led to the formation of bigger particles and a decreased rate of drug release. Moreover, the researchers observed that the speed of the screw, similar to temperature, impacts the rate of flow and therefore the duration for which the material remains in the extruder. The interaction between these factors highlights the crucial need of selecting the appropriate screw speed to balance process parameters and get the required material characteristics ²². Another study conducted by Reitz et al. found that increasing screw speed in a twin screw extruder enhanced convection, resulting in faster mixing and shorter material residence time in the extrusion barrel ²³.
Crowley et al. discovered that a reduced screw speed resulted in polymer breakdown during the processing of polyethylene oxide containing chlorpheniramine (CPM) in a twin-screw extruder.
Prolonged residence time and prolonged exposure to the temperature of extrusion resulted in deterioration. With the increase in screw speed, the viscosity of the polymer melt decreased until the point of melt fracture was attained. Nevertheless, increased speeds resulted in polymer deterioration caused by heat generated from the mechanical power of the screw. The screw speed must be calibrated for each application, as it affects multiple factors in the process of extrusion. If the goal is amorphization, a high screw speed is necessary to provide significant shear mixing with a brief residence period. Conversely, to attain high purity cocrystals, it is necessary to decrease the screw speed to enhance the residence time and, subsequently, the mixing duration.
A crucial factor is the significant correlation between both the feeding rate and the screw speed. When augmenting the extrusion process, an increase in screw speed requires an adjustment in the feed rate to provide a consistent melt flow. ²⁴
Feeding
The feed rate and screw speed in an extruder affect the fill level of the barrel. Varying the feed rate while maintaining the screw speed maintains the filling rate, as increasing the feed rate increases the filling rate. Almeida et al., suggested a balance between feed rate and screw speed for constant melt flow²⁴. The filling percentage typically ranges from 20 to 55% and can be calculated by following:

Where, FR is the rate of feed (g/min), RTD is mean time of residence (min), ρ is bulk density of polymer/mix (g/ml) and Vfree free volume in the extruder (mL).
Gosh et al. performed additional research on the effects of feeding rate and modification on the extrusion of a thermolabile substance. They found that materials can be fed into the extruder barrel in two ways: starve fed (controlled by the feeder) or flow fed (controlled by the screws), with starve fed materials having a feeding rate independent of screw speed. If the material is flow-fed, the throughput is regulated by the screws. Nevertheless, alterations in feeding rates do not consistently impact the quality of the result²¹. Verreck et al. found that the solid condition of itraconazole (ITZ) remained unaffected when it was extruded with HPMC with a feed rate ranging from 2kg/h to 1kg/h. The researchers discovered that regardless of the feed rate (2 kg/h, 1.5 kg/h, or 1 kg/h), amorphous extrudates of ITZ were consistently formed²⁵. Verhoeven et al. conducted an experiment where they introduced a combination of ethylcellulose and MPT (Metoprolol Tartrate) into a twin-screw extruder at different feed rates of 6 g/min, 25 g/min, and 50 g/min. They found that the feed rate had no impact on the quality of the extrudate or its ability to dissolve ²⁶. Similarly, Saerens et al. showed that changing the feed rate of the MPT/Eudragit1 RS PO mixture from 0.325 kg/h to 0.275 kg/h did not affect the expected MPT concentration values. Ultimately, it is crucial to consider that the speed of the screw must be matched with the rate at which the material is being fed. This correlation will establish the optimal filling level of the barrel ²⁷. In a twin-screw extruder, it is recommended to load the barrel at around 20-50% capacity, compared to 70-100% in a single-screw extruder ²⁸. This will significantly affect the torque of the screws and the amount of time the mixture stays in the barrel. Increasing the feeding rates will result in greater torque values and a shorter residence period.
Materials used for extrusion.
Hot melt extruded dosage forms require materials that comply with the same stringent safety and purity standards as those used in conventional pharmaceutical formulations. The ingredients employed in their production are commonly utilized in a variety of solid dosage forms, including pellets, tablets, and transdermal systems. These materials must exhibit sufficient thermal stability to withstand the demands of the extrusion process. Hot-melt extruded dosage forms comprise intricate combinations of APIs and functional excipients, which are classified into bulking agents, release-modifying agents, matrix carriers, and various additives. Excipients play a pivotal role in imparting specific properties to melt-extruded formulations, akin to their functions in traditional dosage forms ²⁹.
The characteristics of active pharmaceutical ingredients frequently regulate the preparation, and formulation substitutes available to pharmaceutical scientists for developing a suitable dosage form. Melt-extrusion represents an innovative technology within the pharmaceutical sector, providing advantages compared to conventional processing methods. The process is inert, thereby preventing potential drug degradation caused by hydrolysis. Furthermore, materials with poor compaction properties can be integrated into tablets by reducing an extruded rod, thereby resolving tableting issues associated with conventional compressed dosage forms. The active ingredient must exhibit thermal stability for melt-extrusion, necessitating an initial evaluation of its chemical, thermal, and physical properties. The drug can exist as an undissolved solid dispersion or be fully dissolved in the polymer in the final dosage form, influencing the processability and stability of the product ³⁰. Besides temperature degradation, the active component could hinder the functionality of formulation components. Oxprenolol hydrochloride was observed to undergo melting during the melt extrusion process, resulting in a reduction in the viscosity of the extrudate and yielding material with suboptimal handling properties ³¹. In related research, Cuff and Raouf reported that fenoprofen calcium delayed the solidification of a polyethylene glycol (PEG) and microcrystalline cellulose (MCC) matrix during injection moulding, which adversely affected the product quality ³². Additionally, another study demonstrated that lidocaine effectively reduced the glass transition temperature of Eudragit E/high-density polyethylene (HDPE) films ³³. More recently, hydrocortisone was found to exhibit a time-dependent decrease in the glass transition temperature of hydroxypropyl cellulose (HPC) films, highlighting its impact on material properties over time. ³⁴.
The selection of polymers for the hot-melt extrusion (HME) process is primarily influenced by factors such as polymer-drug interactions, stability, miscibility, and the intended functionality of the final dosage form. Numerous carriers (polymers) have been studied or utilized in the development of HME-based formulations, as outlined in Table 3.
The selection of a suitable plasticizer for hot-melt extrusion (HME) systems is primarily governed by considerations of stability and compatibility. Citrate esters, triacetin, and low-molecular-weight polyethylene glycols (PEGs) have been extensively studied as plasticizers in HME formulations. Plasticizers play a critical role in lowering the glass transition temperature and processing temperature of polymers, thereby enhancing the stability of both the (API) and the polymer carrier. They also reduce the shear forces required for extrusion, facilitating the processing of high-molecular-weight polymers. It is essential to evaluate the thermal-chemical volatility and stability of the plasticizer during production and storage. The materials used in HME are consistent with those employed in conventional pharmaceutical manufacturing methods. While the thermal stability of the compounds is critical, some thermolabile molecules may still pose challenges due to the relatively short processing times involved.
The incorporation of a plasticizer can significantly lower the processing temperatures required for HME, thus reducing the risk of degradation of both the carrier and the drug. Furthermore, the dissolution rate of the active ingredient can be modulated—either increased or decreased—depending on the properties of the rate-modifying agent utilized ³⁵⋅³⁶.
Table 3: Polymers commonly used in hot melt extrusion technology.
Polymer | Structure | Glass transition temperature | Applications | Advantages | Disadvantages |
Polyethylene Glycol (PEG) |  | -60°C to -10°C | Solid dispersions, controlled release formulations | Enhances plasticity, improves drug solubility | Low thermal stability, can cause drug recrystallization |
Polyvinylpyrrolidone (PVP) |  | 150°C | Solid dispersions, bioavailability enhancement | Strong hydrogen bonding, excellent film former | Hygroscopic, sensitive to moisture |
Ethylcellulose (EC) |  | 130°C - 150°C | Matrix systems, film coatings | Water-insoluble, good mechanical strength | High processing temperatures required |
Eudragit (Methacrylic Copolymers) |  | 50°C - 150°C | Enteric coatings, controlled release | Wide variety of grades for specific applications | High sensitivity to process conditions |
Hydroxypropyl Cellulose (HPC) |  | 70°C - 110°C | Immediate or controlled release formulations | Excellent binding properties, water-soluble | Low mechanical strength |
Polycaprolactone (PCL) |  | -60°C | Long-term release systems, biodegradable scaffolds | Biodegradable, low melting temperature | Slow degradation rate |
Polylactic acid (PLA) |  | 55°C - 60°C | Drug delivery systems, biodegradable scaffolds | Biocompatible, biodegradable | Brittleness, needs plasticizers for flexibility |
Polyvinylpyrrolidone-Vinyl Acetate (PVP-VA Copolymer) |  | 150 to 180 °C | Solid dispersions, film-forming agent | Good solubility in organic solvents, enhances drug release | Sensitive to moisture, moderate thermal stability |
Polyethylene Oxide (PEO) |  | 60°C - 70°C | Extended-release formulations, matrix systems | Good swelling, high molecular weight | Sensitive to thermal degradation |
Formulation approaches using Hot Melt Extrusion
HME facilitates the preparation of solid dispersions, enhancing the solubility and bioavailability of poorly water-soluble drugs. It also plays a pivotal role in the development of controlled-release (CR) and sustained-release (SR) formulations, ensuring precise drug delivery over an extended period.
Additionally, HME has enabled advancements in 3D (Three dimensional) printing, allowing the creation of customized dosage forms and complex drug delivery systems. Furthermore, its integration with lipid-based formulations has unlocked new possibilities for improving the stability and delivery of lipophilic drugs. The following sections delve deeper into each of these applications, highlighting their principles, benefits, and challenges as shown in Figure 2.
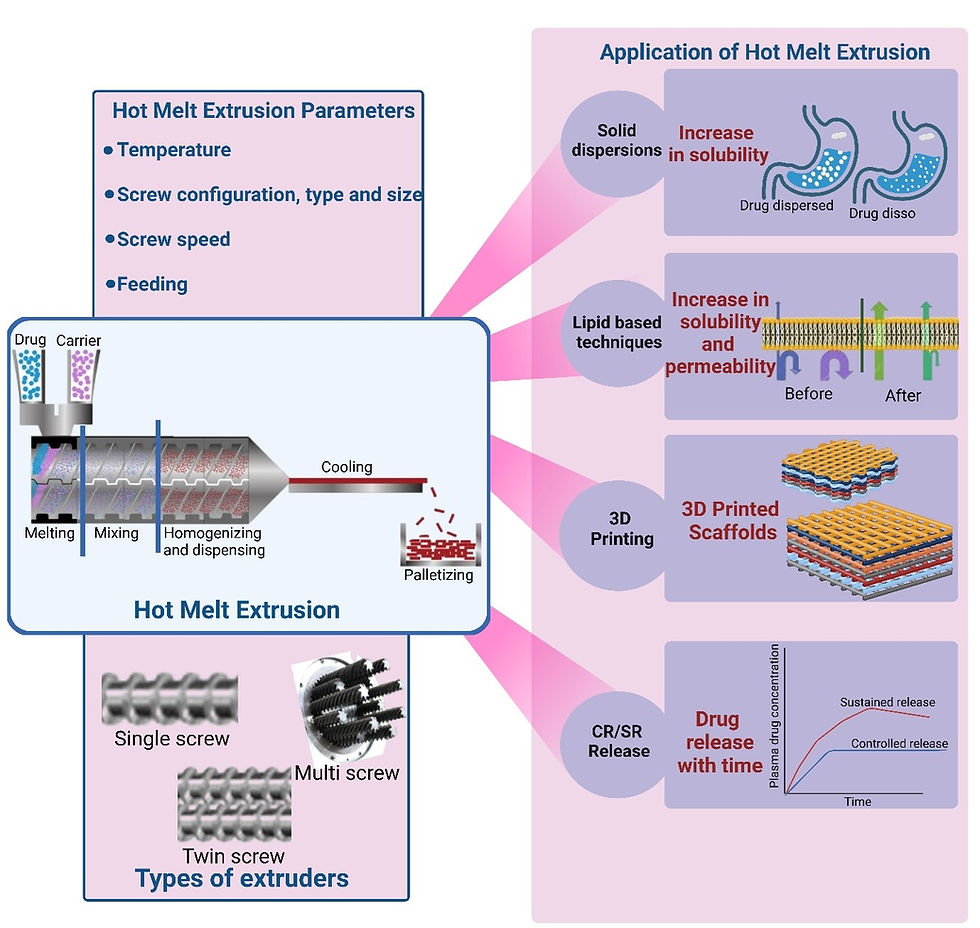
Hot Melt Extrusion and Solid dispersions (SDs)
Solid dispersion (SD) is a technique in which APIs are incorporated within a hydrophilic inert carrier matrix. It is used to transform an API into an amorphous state, hence enhancing its solubility. Melt fusion and solvent evaporation techniques can be used to prepare solid dispersions but HME is an appropriate method for solid dispersions, as it eliminates the need for solvents, hence reducing stability risks throughout the formulation's shelf life. Considering the HME process setup and temperature of processing, the twin type screw extruder is becoming the preferred alternative in the pharmacy for producing solid dispersions ³⁷. HME using TWE is the most feasible method in the pharmaceutical sector for the formulation of SD. It is readily scalable, economical, and environmentally sustainable, rendering it a preferred alternative for solvent-based solid dispersion preparation methods such as spray drying. The removal of the solvents in HME makes it an eco-friendly and sustainable process.
Hulsmann et al. employed the HME for enhancing the rate of dissolution of 17β-oestradiol hemihydrate, a drug with low water solubility. Excipients, included PVP, PEG 6000, and a vinylpyrrolidone-vinyl acetate copolymer, along with the addition of Gelucire 44/14 and Sucroester WE15. The SDs markedly enhanced solubility, achieving a 30-fold increase with the sustained dissolution behaviour of the tablet dosage form ³⁸.
Lee et al conducted a study which aimed to improve the therapeutic efficacy of bisacodyl by formulating solid dispersions using hot-melt extrusion as shown in Figure 2. The hydroxypropyl methylcellulose (HPMC)-based solid dispersions were chosen due to their superior physical stability and inhibitory effects on precipitation. Enteric-coated tablets with HPMC-bisacodyl at a 1:4 ratio shows improvement in in-vivo therapeutic laxative efficacy in rabbits induced by constipation compared to un-solubilized raw bisacodyl preparations ³⁹.

He et al. prepared a SD of drug-fenofibrate with good bioavailability using the HME process and compared the dissolution profiles of PVP-VA S630 and Eudragit® E100. The SD was formulated with Eudragit E100 or PVP-VA and characterized through DSC, in vivo bioavailability assessment, in vitro dissolution testing, and X-ray diffraction. The study result suggests that the drug-fenofibrate existed in a non-crystalline state, leading to the finding that HME is a successful method for enhancing fenofibrate solubility and bioavailability ⁴⁰.
Wang et al. demonstrated that hot-melt extrusion (HME) technology effectively enhances the oral bioavailability and dissolution rate of Ginkgo biloba extract solid dispersions (GBE-SD). In an in vivo study conducted on male Sprague-Dawley rats, the results indicated a significant increase in the Cmax and the AUC following the oral administration of GBE-SD. These findings suggest that HME is a promising manufacturing approach for developing solid dispersions of natural product compounds, offering improved oral bioavailability and dissolution characteristics ⁴¹.
Alshahrani et al. employed hot melt extrusion (HME) technology to formulate solid dispersions of carbamazepine (CBZ) utilising Soluplus® & hydroxypropyl methylcellulose acetate succinate (HPMCAS-HF). This combination enhanced both the solubility as well as the stability of the formulated solid dispersions. The solid dispersions were analyzed using thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectroscopy. The results indicated that HPMCAS-HF, Soluplus®, and CBZ maintained stability throughout extrusion processing conditions. Increased drug concentrations led to the resurgence of CBZ crystalline peaks. HPMCAS-HF enhanced hydrogen bonding intensity, collaborating synergistically with Soluplus® to improve the solubility and stability formulation ⁴².
Dedroog et al. demonstrated that HME is an effective technique for achieving high levels of drug loading in solid-state drug SD formulations, surpassing both spray drying and cryo-milling procedures. The study with polymers and drug in different proportions demonstrated that increasing drug loading is dependent upon the production process and the type of carrier used ⁴³. Tian et al. successfully formulated solid dispersions with increased drug loading using Eudragit® E, demonstrating that hot-melt extrusion (HME) can achieve drug concentrations of 60%–70% within the formulation. The enhanced drug loading capacity and stability were attributed to strong molecular interactions, which serve as a critical foundation for the efficacy of the HME process ⁴⁴.
Hot Melt Extrusion and SR/CR formulations
A study by Verhoeven et al. used hot-melt extrusion (HME) to create mini-matrices for sustained drug release, using ibuprofen as the model drug and ethylcellulose as the SR agent along with xanthan gum to improve the drug release profile. The release mechanism was diffusion-driven, with swelling playing a significant role. A crossover study on dogs showed sustained ibuprofen release compared to immediate-release and other SR formulations, with minimal bioavailability differences between 20% and 30% xanthan gum matrices ⁴⁵.
Ozguney et al. developed mini matrices (extended release) using HME process with a twin-screw type of extruder, employing theophylline and ibuprofen as model drugs, with Kollidon® SR serving as the carrier. The maximum torque and glass transition temperature exhibited an inverse relationship with (Ibuprofen) IBU concentration, indicating a plasticizing effect. Also, the percentage of drug loading of both APIs is significantly influenced by the drug release rate. Furthermore, elevating the extrusion temperature resulted in a reduced release rate of hot melt extruded theophylline while enhancing the release rate of IBU. The study substantiated the notion that IBU had a plasticizing effect on Kollidon® SR ⁴⁶.
Almeida et al. demonstrated that ethylene vinyl acetate (EVA) and polyethylene oxide (PEO) polymers are effective in sustaining the release of active pharmaceutical ingredients (APIs) from hot melt extruded (HME) matrices. Using EVA samples with varying vinyl acetate (VA) contents, meloxicam as a model drug (MPT), and PEO as a swelling agent, EVA/MPT/PEO formulations were successfully prepared through HME. The drug release profiles, solid-state properties, porosity, and polymer miscibility were comprehensively analyzed using differential scanning calorimetry (DSC), X-ray tomography, and Raman spectroscopy. Advanced Raman and near-infrared (NIR) spectroscopy techniques revealed molecular-level interactions between EVA and the drug, offering a deeper understanding of the material behaviour and its impact on drug release dynamics and stability ²⁴.
Controlled release formulations aim to deliver drugs at a predetermined rate for a specified period, improving therapeutic outcomes and patient compliance. These formulations allow the drug to be released slowly, prolonging its action in the body and reducing dosing frequency. HME technology is widely applied in the development of such controlled-release systems due to its ability to manipulate drug release profiles through various formulation strategies.
Li et al. investigated the application of hot-melt extrusion (HME) in the development of dexamethasone-loaded implants utilizing polymers such as polylactic acid (PLA), PEG-PPG-PEG copolymer, and Pluronic F68. Differential scanning calorimetry (DSC) was employed to assess the compatibility between the drug and implant materials, while mass loss measurements and scanning electron microscopy (SEM) were utilized to evaluate the degradation behaviour of the implants. The implants demonstrated an encapsulation efficiency of 98% and a drug loading capacity of 49%. In vitro studies revealed that the implants sustained a controlled drug release for approximately 121 days. Additionally, the degradation rate of the implant device was notably accelerated in alkaline environments, highlighting the influence of pH on the degradation kinetics. These findings underscore the potential of HME technology in creating long-acting implantable drug delivery systems with controlled release properties. ⁴⁷.
Fukuda et al. investigated the influence of sodium bicarbonate (NaHCO₃) on the physicochemical properties of hot-melt extruded (HME) controlled-release tablets formulated with chlorpheniramine maleate, acetohydroxamic acid, or a combination of both, alongside polymers such as Eudragit® RS PO, Eudragit® E PO, or their mixture. The study found that the addition of NaHCO₃ did not significantly alter the drug release rate. However, the incorporation of NaHCO₃ reduced the density of the HME tablets to below 1 g/cm³, enabling buoyancy. The porous internal morphology of the tablets was attributed to the generation of carbon dioxide (CO₂) gas in 0.1 N HCl, which enhanced their buoyant properties. This increased buoyancy was linked to the thermal decomposition of NaHCO₃ within the acrylic polymer matrix during the HME process, contributing to the tablets' functional characteristics. ⁴⁸.
Miyagawa et al. fabricated controlled release granules of diclofenac with carnauba wax and rate-controlling components using a twin-screw extruder in a high-pressure moulding (HME) process. The wax matrix showed significant mechanical strength with sustained drug release from the granules ⁴⁹⋅⁵⁰. Sato and Miyagawa prepared wax matrix tablets using HPMC, Eudragit® L-100, and sodium chloride by HME (employing TSE) to modulate dissolution rates. The study reported reduced processing temperatures, improved mixing and dispersing capabilities, and shorter residence duration by using TSE ⁵¹.
Maniruzzaman et al. studied the effect of control release polymer/lipid formulations on the dissolution rates of water insoluble indomethacin (INM) co-processed by hot melt extrusion. The formulations, consisting of hydrophilic hydroxypropyl methyl cellulose polymer (HPMCAS) and stearoyl macrogol-32 glycerides—Gelucire 50/13 (GLC), were processed using a twin-screw extruder. X-ray powder diffraction, differential scanning calorimetry, and HSM analysis revealed the presence of amorphous INM within the matrices. The HPMC grade polymer presented a predominant effect, and no release was observed in alkaline media, but a controlled release was observed in acid media showing an increased dissolution rates of indomethacin.

Hot Melt Extrusion and 3D Printing
Three-dimensional printing (3D printing) technologies, including extrusion based printing, light based printing, and droplet based printing methods, are investigated for the production of different pharmaceutical formulations ⁵². Spritam® by Aprecia Pharmaceuticals is the 1st USFDA approved 3D printed tablet comprising levetiracetam for the management of seizures in epilepsy, employing the Zipdose technology (powder bed binding method. Extrusion-based systems include FDM (fused deposition modeling), PAM (pressure-assisted microsyringes, MJS (multiphase jet solidification, and PED (precision extrusion deposition). In extrusion-based 3D printing, the ingredients, in any state (molten or liquid), are extruded with pressure. The filament which is extruded is 3D printed into the specified form. FDM with HME aims to manufacture filament with certain characteristics for 3D printing and is a registered trademark of Stratasys® ⁵².
Unlike FDM, PAM facilitates 3D printing at ambient temperature, and eliminates the need for pre-thermal processing in 3D printing, which is advantageous for constructing systems that incorporate thermosensitive pharmaceuticals ⁵³. In the PAM 3D printing process, a viscous paste or semi-liquid substance is extruded from a syringe by force or pressure to generate a three-dimensional printed structure ⁵². MJS is a method that uses heated paste extrusion through a jet nozzle to incrementally build a 3D form layer by layer. ⁵⁴. Unlike the conventional FDM approach that necessitates precursors, PED technology operates without filaments and can use materials in pulverised and pellet form to produce the required 3D object ⁵⁵. PED has demonstrated more significance in the pharmacy when compared to FDM and MJS 3D printing techniques. Current PED uses a single screw to convey and distribute materials through three-dimensional movement to generate objects. Over time, researchers can synergistically utilise the advantages of both HME and PED to attain the desired 3D printed dosage form ⁵².
Borujeni et al. prepared 3DP matrix tablets of carbamazepine (CBZ) by fused deposition modeling using hydroxypropyl cellulose, ethyl cellulose, and triethyl cellulose to achieve zero-order kinetics. The study revealed that a correct concentration of plasticizer is crucial for smooth 3D printing. ⁵⁶⋅⁵⁷. The combination of HME and 3D printing technologies can address drug side effects and improve drug delivery. A study by Tan et al. introduced a polymeric SR formulation called PrintCap, which uses HME and FDM 3D printing techniques. The balance between HPC and Eudragit content was measured to produce filaments suitable for 3D printing. 3D printed caplets can be transformed into on-demand drug delivery systems for sustained release ⁵⁸. Several 3D manufactured sustained release and prolonged release tablets were evaluated, yielding comparable results ⁵⁹⁻⁶¹. This integration of HME with 3D printing technology can enable hospitals to produce pharmaceutical drugs and medication on-site, according to patient needs.
Verstraete et al. prepared a high-drug-loaded dosage form using hybrid technology, incorporating filaments into tablets with a high drug loading and significant hardness. The 3D printed tablets showed superior release kinetics compared to injection-moulded tablets. ⁶² Xu et al. addressed the lack of quantitative characterisation methods for filament printability in a Fused Deposition Modelling (FDM) application by examining texture analysis methods and a "Toughness" parameter. They used semi-solid extrusion to create a five-in-one dose combination polypill and bilayer tablets, offering versatility and accessibility for 3D printing applications ⁶³.
Lee et al. focused on the fabrication of modified-release solid dosage forms using 3D HME as shown in Figure 4. The forms were printed using a 3D printer with an air pressure driving HME system, containing ibuprofen (IBU), polyvinyl pyrrolidone (PVP), and polyethylene glycol (PEG). The physicochemical properties, dissolution rates, and floatable behaviour of the printed forms were evaluated. The dissolution rate of IBU was dependent on the geometric properties of the form and could be modified by merging two different geometries into one. The 3D HME process demonstrated high reproducibility and accuracy in preparing dosage forms ⁶⁴.

Lipid based formulations (LBF)
LBF serve as one of the strategies for bioavailability enhancement. They facilitate sustained release, enable film coatings, mask taste, and improve skin delivery of poorly water-soluble drugs. The predominant approach involves solubilising drugs within a lipid matrix and incorporating excipients such co-solvents, surfactants and co-surfactants to ensure formulation stability and minimise interfacial tension among liquids.
LBF can be manufactured through different extrusion methods tailored to specific formulation needs. Lipid extrusion entails the incorporation of a preblended lipid-drug mixture, along with additional components, into a feeder, followed by processing through an SSE or TSE. The resulting homogeneous dispersion may consist of solid lipid extrudate, or liquid emulsions derived from soft lipids. The screw design is optimized through processes such as kneading, blending, consolidation, and transferring the material from the hopper to the die. Keen et al. encountered difficulties in processing Kollidon 30 at temperatures below 180 °C, attributed to its high viscosity, which results in elevated torque of the screws. The addition of Compritol® 888 ATO resulted in a reduction of torque load from 425N to 125N. Solid lipid extrusion (SLE) is a method for extruding lipid solids in a solid matrix type structure or mass without the need for melting the lipids. SLE processes lipids at temperatures below their melting range, resulting in a stable release (CR/SR) system. Thermo-mechanical treatment can induce plasticity in lipids. The material is extruded using a die of designated diameter, yielding various shapes. ⁶⁵.
LBF has been extensively used in applications including taste masking, lipid implants, oral-dispersible formulations, modified release formulations, and GRDDS (Gastro- retentive drug delivery systems). Compritol 888 ATO serves as a controlled release agent, Precirol ATO 5 serves as a taste masking agent, Gelucire 50/13 is used for poorly water-soluble drugs, and stearic acid (SA) functions as pH-dependent release. The melting technique has been investigated for the preparation of formulations owing to the thermal stability of lipids at elevated temperatures. This technique provides benefits including high scalability, environmental sustainability, reduced oxygen exposure, time efficiency, improved process control, and enhanced uniformity. A comparative study demonstrated that lipid extrusion resulted in a more rapid drug release than both direct compression and prilling methods ⁶⁶. A combination of behenic, myristic acid and SA exhibited rapid release of drugs for lipid extrudates in comparison to prill, as evidenced by cross sections of X-ray tomography of the lipid-drug matrices revealing a uniform pore distribution ⁶⁷. HME has also emerged as a versatile and efficient technique for formulating various lipid-based drug delivery systems. It is increasingly applied in the production of solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), lipid-based granules, and lipid implants, offering enhanced stability and controlled drug release.
SLNs consist of a solid lipid core that is surrounded by surfactants or emulsifiers on their surfaces. SLNs exhibit advantages relative to emulsions, polymeric nanoparticles, and liposomes. Common techniques for the preparation of SLNs include high-pressure homogenisation, spray drying, solvent evaporation/emulsification, and supercritical fluid (SCF) technologies. These SLNs are formulated using high molecular weight lipids known for their excellent coating properties, which contribute to the formation of smooth particle surfaces and enhanced dispersibility. Patil et al. studied the impact of barrel lipid temperature, surfactant composition, and screw speed on fenofibrate particle size and entrapment efficiency. They found that higher lipid concentrations increased particle size and improved drug entrapment efficiency. Reduced screw speeds led to longer residence time for solid lipid nanoparticles (SLNs) within the barrel, reducing particle size due to prolonged interaction between surfactants and melted lipid. The study aimed to optimize SLN production to achieve sub-200 nm particle size and enhanced drug entrapment by increasing residence time and high shear mixing ⁷.
Lipidic granules (LG) are formed via melt aggregation and agglomeration and/or melt pelletization, resulting in excellent sphericity and a narrow particle size range. LG are formulated with thermoplastic binders such as polyethylene, polyethylene-co-ethyl acrylate, and hydroxypropyl cellulose, polystyrene. The LG undergo processing at elevated temperatures to create liquid bridges between particles during nucleation and growth. Traditional techniques encompass high shear mixing, fluid bed processing, and melt pelletization. Keen et al. formulated granules via hot melt extrusion for controlled-release tramadol HCl tablets utilising glyceryl behenate as a controlled-release substance and binding agent. Another study uses a HME barrel which is shown to increase lipidic percentage that led to larger lipid droplets and an increase in size of granules. The reduction in particle size following extrusion may result from the milling operation within the twin-screw extruder. The increase in particle size distribution is inversely related to feed rate, indicating the need for regulation of feed rate during HME ⁶⁸.
NLCs consist of a combination of liquid lipids and solid lipids that is formulated into a dispersed system for API carrier. NLCs provide benefits compared to solid-liquid carriers (SLNs), notably adjustable drug release, superior dispersion capacity, improved stability, and greater capacity of drug loading. NLCs are extensively used in formulations for poorly water-soluble pharmaceuticals by methods such as spray drying, emulsion-evaporation, and high-pressure homogenization. A recent study developed a lipidic system for lidocaine carrier using specific lipids, extruded via hot melt extrusion (HME), and subjected to probe sonication to form an emulsion with CR properties. Lipids have been used as carrier system for SR polymers to facilitate regulated drug delivery ⁶⁹. Silva et al. conducted a study in which a HPMCAS polymer was blended with a molten mass of surfactant and lipid for prolonged release ⁷⁰.
Chemotherapeutic drugs are frequently extremely hydrophobic and possess short half-lives, impacting their therapeutic efficacy and frequency of dose. Lipid-based formulations, including nanosuspensions and 5-fluorouracil-loaded liposomes, have been developed to enhance distribution and antitumour efficacy. Lipid implants were recently fabricated using HME for the sustained drug administration of tyrosinase-related protein 2 (TRP 2) in tumour therapy. The materials used for extrusion comprised Cholesterol, soybean lecithin, Quil-A, Dynasan 114, and TRP2 peptide/ovalbumin. The technical stress experienced during HME process did not influence the polymorphism state of the lipids. Nevertheless, the extrusion temperature and pressure modified the internal morphology and porosity of the extrudates, influencing the release of TRP2/OVA (Ovalbumin). The extruded lipidic implants demonstrated gradual release (in vitro), indicating its potential for preventative cancer therapy. HME enables the uniform dispersion of APIs within lipid matrices, creating formulations with improved bioavailability and solubility of poorly water-soluble drugs. The thermal and mechanical forces in HME also facilitate the production of complex lipid-based systems without the need for solvents, making it an environmentally friendly and scalable option for advanced drug formulations.
This study aimed to develop continuous and scalable itraconazole PEGylated nano-lipid carriers (ITZ-PEG-NLC) for inhalation delivery of pulmonary aspergillosis. The formulation was successfully formulated, with a drug entrapment efficiency of 97.28 ± 0.50%. In vitro cytotoxicity studies showed a burst-release pattern with a drug release of 41.74 ± 1.49% in 60 minutes. These findings suggest that HME technology could be used to produce continuous scalable ITZ-PEG-NLC as shown in Figure 6.
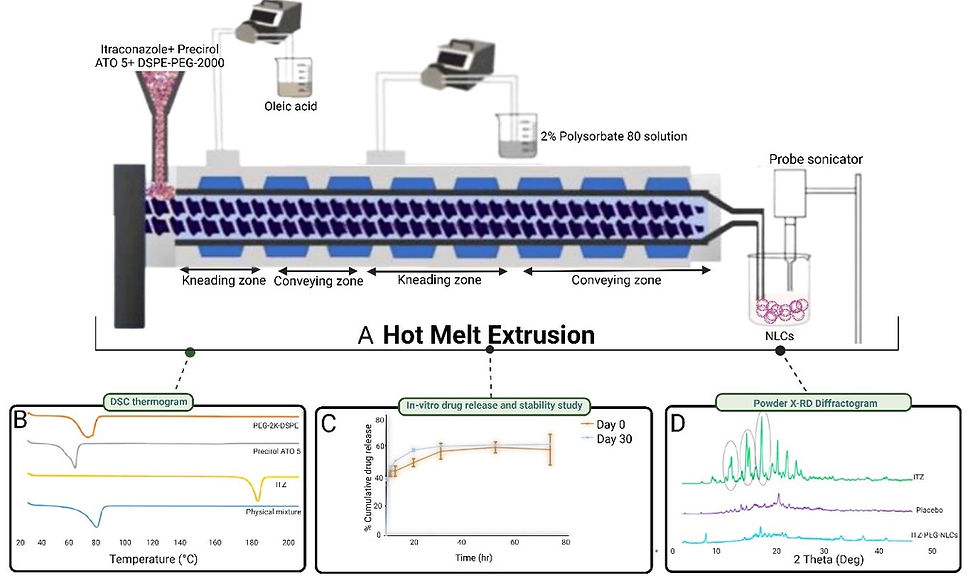
Marketed formulations using HME techniques.
The interest in HME has grown significantly, with over five hunderd papers published in literature in the past decade. HME is being researched to produce various drug delivery systems eg., oral, implants. Despite the potential for formulating poorly soluble drugs into formulations, few have been commercialised thus far. These are summarised in Table 4.
Table 4: Different marketed formulations using Hot melt extrusion technology.

CONCLUSIONS
HME has become a revolutionary technique in manufacturing pharmaceuticals, providing solutions for improving bioavailability, solubility, and controlled drug release. The study emphasizes the adaptability of HME, encompassing solid dispersion preparation, sustained-release formulations to sophisticated 3D printing, and lipid-based systems. The capacity of technology for easy integration with contemporary production methods highlights its scalability and versatility for various pharmaceutical applications.
Notwithstanding limitations such as elevated operational temperatures and limited excipients, persistent research initiatives are addressing these obstacles, hence expanding the use of HME. HME aligns with regulatory frameworks like the FDA's Process Analytical Technology, enhancing formulation techniques of drugs while ensuring quality and sustainability. Upcoming advancements in polymer science and process engineering are expected to optimize the capabilities of HME, facilitating the development of personalized and complex drug delivery systems.
Abbreviations
HME: Hot Melt Extrusion
FDA: Food and Drug Administration
PAT: Process Analytical Technology
BCS: Biopharmaceutical Classification
CE: Cold extrusion
SSE: Single screw extruders
TSE: Twin screw extruders
MSE: Multi screw extruders
API: Active Pharmaceutical Ingredient
DSC: Differential scanning calorimetry
XRD: X-ray diffraction
EC: Ethylcellulose
HPMCAS: Hydroxypropyl Methylcellulose Acetate Succinate
PEO: Polyethylene oxide
ITZ: Interfacial Transition Zone
CPM: Chlorpheniramine
ITZ: Itraconazole
MPT: Metoprolol Tartrate
PEG: Polyethylene Glycol
MCC: Microcrystalline cellulose
HPC: Hydroxypropyl cellulose
HDPE: High-Density Polyethylene
PCL: Polycaprolactone
PLA: Polylactic acid
PVP-VA: Polyvinylpyrrolidone-Vinyl Acetate
PEO: Polyethylene Oxide
CR: Controlled release
SR: Sustained release
3D: Three dimensional
SDs: Solid dispersions
HPMC: Hydroxypropyl methyl cellulose
GBE-SD: Ginkgo biloba extract solid dispersions
CBZ: Carbamazepine
HPMCAS-HF: Hydroxypropyl Methylcellulose Acetate Succinate
ATR-FTIR: Attenuated total reflectance Fourier-transform Infrared
IBU: Ibuprofen
EVA: Ethyl vinyl acetate
VA: Vinyl acetate
NIR: Near infrared
PEG-PPG-PEG: Polyethylene glycol- Polypropylene glycol- Polyethylene glycol
NaHCO₃: Sodium bicarbonate
INM: Indomethacin
GLC: Gelucire
FDM: Fused deposition modeling
PAM: Pressure assisted microsyringes
MJS: Multiphase jet solidification
PEG: Precision extrusion deposition
SLE: Solid lipid extrusion
GRDDS: Gastro retentive drug delivery system
SA: Stearic acid
SCF: Supercritical fluid
SLNs: Solid lipid nanoparticles
LC: Lipidic granules
NLCs: Nanostructured Lipid Carriers
TRP 2: Tyrosinase-related protein 2
OVA: Ovalbumin
ITZ-PEG-NLCs: Itraconazole PEGylated nano-lipid carriers
References
01. Bhalani DV, Nutan B, Kumar A, Singh Chandel AK. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines. 2022;10(9).
02. Tambe S, Jain D, Agarwal Y, Amin P. Hot-Melt Extrusion: Highlighting Recent Advances in Pharmaceutical Applications. Journal of Drug Delivery Science and Technology. 2021.
03. Hessel V, Mukherjee S, Mitra S, Goswami A, Tran NN, Ferlin F, et al. Sustainability of flow chemistry and microreaction technology. Green Chemistry. 2024;26(18):9503-28.
04. Jones D, Andrews G. ChemInform Abstract: Formulation and Characterisation of Hot Melt Extruded Dosage Forms: Challenges and Opportunities. ChemInform. 2010;41.
05. Tan DK, Davis DA, Jr., Miller DA, Williams RO, 3rd, Nokhodchi A. Innovations in Thermal Processing: Hot-Melt Extrusion and KinetiSol® Dispersing. AAPS PharmSciTech. 2020;21(8):312.
06. Li X, Hong X, Shuai S, Han X, Li C, Zhang H, et al. A review of hot melt extrusion technology: Advantages, applications, key factors and future prospects. Journal of Drug Delivery Science and Technology. 2024;98:105884.
07. Patil H, Tiwari RV, Repka MAJAP. Hot-melt extrusion: from theory to application in pharmaceutical formulation. AAPS PharmSciTech. 2016;17:20-42.
08. Luker KJHMEPA. Single‐Screw Extrusion: Principles. Pharmaceutical Applications. 2012:1-21.
09. Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33(9):909-26.
10. Thiele W. Twin-screw extrusion and screw design. Pharmaceutical extrusion technology: CRC Press; 2018. p. 71-94.
11. Loukus J, Halonen A, Gupta M, editors. Elongational flow in multiple screw extruders. ANTEC-CONFERENCE PROCEEDINGS-; 2004: UNKNOWN.
12. Repka MA, Langley N, DiNunzio JJM, Technology, Design DP. Melt extrusion. Springer. 2013;4(5).
13. Breitenbach J. Melt extrusion: from process to drug delivery technology. European Journal of Pharmaceutics and Biopharmaceutics. 2002;54(2):107-17.
14. Nikitine C, Rodier E, Sauceau M, Fages J. Residence time distribution of a pharmaceutical grade polymer melt in a single screw extrusion process. Chemical Engineering Research and Design. 2009;87(6):809-16.
15. Jijun F, Lili Z, Tingting G, Xing T, Haibing H. Stable nimodipine tablets with high bioavailability containing NM-SD prepared by hot-melt extrusion. Powder Technology. 2010;204(2):214-21.
16. Almeida A, Possemiers S, Boone MN, De Beer T, Quinten T, Van Hoorebeke L, et al. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. European Journal of Pharmaceutics and Biopharmaceutics. 2011;77(2):297-305.
17. Chokshi R, Zia HJIjopr. Hot-melt extrusion technique: a review. Iranian journal of pharmaceutical research. 2004;3(1):3-16.
18. Nakamichi K, Nakano T, Yasuura H, Izumi S, Kawashima Y. The role of the kneading paddle and the effects of screw revolution speed and water content on the preparation of solid dispersions using a twin-screw extruder. International journal of pharmaceutics. 2002;241(2):203-11.
19. Verhoeven E, De Beer TRM, Van den Mooter G, Remon JP, Vervaet C. Influence of formulation and process parameters on the release characteristics of ethylcellulose sustained-release mini-matrices produced by hot-melt extrusion. European Journal of Pharmaceutics and Biopharmaceutics. 2008;69(1):312-9.
20. Lang B, McGinity JW, Williams RO, III. Dissolution Enhancement of Itraconazole by Hot-Melt Extrusion Alone and the Combination of Hot-Melt Extrusion and Rapid Freezing—Effect of Formulation and Processing Variables. Molecular pharmaceutics. 2014;11(1):186-96.
21. Ghosh I, Vippagunta R, Li S, Vippagunta SJPD, Technology. Key considerations for optimization of formulation and melt-extrusion process parameters for developing thermosensitive compound. Pharmaceutical Development and Technology. 2012;17(4):502-10.
22. Reitz E, Vervaet C, Neubert RH, Thommes MJEJoP, Biopharmaceutics. Solid crystal suspensions containing griseofulvin–preparation and bioavailability testing. European Journal of Pharmaceutics and Biopharmaceutics. 2013;83(2):193-202.
23. Reitz E, Podhaisky H, Ely D, Thommes M. Residence time modeling of hot melt extrusion processes. European Journal of Pharmaceutics and Biopharmaceutics. 2013;85(3, Part B):1200-5.
24. Almeida A, Saerens L, De Beer T, Remon JP, Vervaet C. Upscaling and in-line process monitoring via spectroscopic techniques of ethylene vinyl acetate hot-melt extruded formulations. International journal of pharmaceutics. 2012;439(1):223-9.
25. Verreck G, Six K, Van den Mooter G, Baert L, Peeters J, Brewster ME. Characterization of solid dispersions of itraconazole and hydroxypropylmethylcellulose prepared by melt extrusion—part I. International journal of pharmaceutics. 2003;251(1):165-74.
26. Verhoeven E, De Beer T, Van den Mooter G, Remon JP, Vervaet CJEjop, biopharmaceutics. Influence of formulation and process parameters on the release characteristics of ethylcellulose sustained-release mini-matrices produced by hot-melt extrusion. European journal of pharmaceutics and biopharmaceutics. 2008;69(1):312-9.
27. Saerens L, Vervaet C, Remon J-P, De Beer T. Visualization and Process Understanding of Material Behavior in the Extrusion Barrel during a Hot-Melt Extrusion Process Using Raman Spectroscopy. Analytical Chemistry. 2013;85(11):5420-9.
28. Sales A, Director M. A Practical Approach to Scale-up from Bench-top Twin-screw Extruders. 2021.
29. McGinity JW, Zhang F, Koleng J, Repka MJAPR. Hot-melt extrusion as a pharmaceutical process. 2001;4:25-37.
30. Swarbrick J. Encyclopedia of Pharmaceutical Technology, pdf.
31. Follonier N, Doelker E, Cole ETJJoCR. Various ways of modulating the release of diltiazem hydrochloride from hot-melt extruded sustained release pellets prepared using polymeric materials. Journal of Controlled Release. 1995;36(3):243-50.
32. Cuff G, Raouf FJPt. A preliminary evaluation of injection molding as a technology to produce tablets. Pharmaceutical technology. 1998;22(6):96-106.
33. Aitken-Nichol C, Zhang F, McGinity JWJPr. Hot melt extrusion of acrylic films. Pharmaceutical research. 1996;13(5):804-8.
34. Repka MA, Gerding TG, Repka SL, McGinity JW. Influence of Plasticizers and Drugs on the Physical-Mechanical Properties of Hydroxypropylcellulose Films Prepared by Hot Melt Extrusion. Drug Development and Industrial Pharmacy. 1999;25(5):625-33.
35. Verreck G, Six K, Van den Mooter G, Baert L, Peeters J, Brewster ME. Characterization of solid dispersions of itraconazole and hydroxypropylmethylcellulose prepared by melt extrusion--Part I. International journal of pharmaceutics. 2003;251(1-2):165-74.
36. Lakshman JP, Cao Y, Kowalski J, Serajuddin AT. Application of melt extrusion in the development of a physically and chemically stable high-energy amorphous solid dispersion of a poorly water-soluble drug. Molecular pharmaceutics. 2008;5(6):994-1002.
37. El-Egakey MA, Soliva M, Speiser PJPAH. Hot extruded dosage forms. I. Technology and dissolution kinetics of polymeric matrices. Pharmaceutica Acta Helvetiae. 1971;46(1):31-52.
38. Hülsmann S, Backensfeld T, Keitel S, Bodmeier RJEJoP, Biopharmaceutics. Melt extrusion–an alternative method for enhancing the dissolution rate of 17β-estradiol hemihydrate. European Journal of Pharmaceutics and Biopharmaceutics. 2000;49(3):237-42.
39. Lee S-K, Ha E-S, Park H, Kang K-T, Jeong J-S, Kim J-S, et al. Preparation of Hot-Melt-Extruded Solid Dispersion Based on Pre-Formulation Strategies and Its Enhanced Therapeutic Efficacy. Pharmaceutics [Internet]. 2023; 15(12).
40. He H, Yang R, Tang XJDd, pharmacy i. In vitro and in vivo evaluation of fenofibrate solid dispersion prepared by hot-melt extrusion. Drug development and industrial pharmacy. 2010;36(6):681-7.
41. Wang W, Kang Q, Liu N, Zhang Q, Zhang Y, Li H, et al. Enhanced dissolution rate and oral bioavailability of Ginkgo biloba extract by preparing solid dispersion via hot-melt extrusion. Fitoterapia. 2015;102:189-97.
42. Alshahrani SM, Lu W, Park J-B, Morott JT, Alsulays BB, Majumdar S, et al. Stability-enhanced hot-melt extruded amorphous solid dispersions via combinations of Soluplus® and HPMCAS-HF. AAPS PharmSciTech. 2015;16:824-34.
43. Dedroog S, Huygens C, Van den Mooter G. Chemically identical but physically different: A comparison of spray drying, hot melt extrusion and cryo-milling for the formulation of high drug loaded amorphous solid dispersions of naproxen. European Journal of Pharmaceutics and Biopharmaceutics. 2019;135:1-12.
44. Tian Y, Jacobs E, Jones DS, McCoy CP, Wu H, Andrews GPJIjop. The design and development of high drug loading amorphous solid dispersion for hot-melt extrusion platform. International journal of pharmaceutics. 2020;586:119545.
45. Verhoeven E, Vervaet C, Remon JPJEjop, biopharmaceutics. Xanthan gum to tailor drug release of sustained-release ethylcellulose mini-matrices prepared via hot-melt extrusion: in vitro and in vivo evaluation. European journal of pharmaceutics and biopharmaceutics. 2006;63(3):320-30.
46. Özgüney I, Shuwisitkul D, Bodmeier RJEJoP, Biopharmaceutics. Development and characterization of extended release Kollidon® SR mini-matrices prepared by hot-melt extrusion. European Journal of Pharmaceutics and Biopharmaceutics. 2009;73(1):140-5.
47. Li D, Guo G, Fan R, Liang J, Deng X, Luo F, et al. PLA/F68/Dexamethasone implants prepared by hot-melt extrusion for controlled release of anti-inflammatory drug to implantable medical devices: I. Preparation, characterization and hydrolytic degradation study. International journal of pharmaceutics. 2013;441(1-2):365-72.
48. Fukuda M, Peppas NA, McGinity JWJJocr. Floating hot-melt extruded tablets for gastroretentive controlled drug release system. Journal of controlled release. 2006;115(2):121-9.
49. Miyagawa Y, Okabe T, Yamaguchi Y, Miyajima M, Sato H, Sunada HJIjop. Controlled-release of diclofenac sodium from wax matrix granule. International journal of pharmaceutics. 1996;138(2):215-24.
50. Miyagawa Y, Sato H, Okabe T, Nishiyama T, Miyajima M, Sunada HJDd, et al. In vivo performance of wax matrix granules prepared by a twin-screw compounding extruder. Drug development and industrial pharmacy. 1999;25(4):429-35.
51. Sato H, Miyagawa Y, Okabe T, Miyajima M, Sunada HJJops. Dissolution mechanism of diclofenac sodium from wax matrix granules. Journal of pharmaceutical sciences. 1997;86(8):929-34.
52. Zhang J, Vo AQ, Feng X, Bandari S, Repka MA. Pharmaceutical Additive Manufacturing: a Novel Tool for Complex and Personalized Drug Delivery Systems. AAPS PharmSciTech. 2018;19(8):3388-402.
53. El Aita I, Breitkreutz J, Quodbach J. On-demand manufacturing of immediate release levetiracetam tablets using pressure-assisted microsyringe printing. European Journal of Pharmaceutics and Biopharmaceutics. 2019;134:29-36.
54. Geiger M, Steger W, Greul M, Sindel M. Multiphase jet solidification. 1994.
55. Wang F, Shor L, Darling A, Khalil S, Sun W, Güçeri S, et al. Precision extruding deposition and characterization of cellular poly‐ε‐caprolactone tissue scaffolds. Rapid Prototyping Journal. 2004;10(1):42-9.
56. Homaee Borujeni S, Mirdamadian SZ, Varshosaz J, Taheri A. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose. 2020;27(3):1573-89.
57. Öblom H, Zhang J, Pimparade M, Speer I, Preis M, Repka M, et al. 3D-Printed Isoniazid Tablets for the Treatment and Prevention of Tuberculosis—Personalized Dosing and Drug Release. AAPS PharmSciTech. 2019;20(2):52.
58. Tan D, Akdag A. Synthesis of New Flexible Coumarin Dimers for Sodium and Potassium Differentiation. Journal of Fluorescence. 2020;30(1):27-34.
59. Korte C, Quodbach J. Formulation development and process analysis of drug-loaded filaments manufactured via hot-melt extrusion for 3D-printing of medicines. Pharmaceutical Development and Technology. 2018;23(10):1117-27.
60. Genina N, Boetker JP, Colombo S, Harmankaya N, Rantanen J, Bohr A. Anti-tuberculosis drug combination for controlled oral delivery using 3D printed compartmental dosage forms: From drug product design to in vivo testing. Journal of Controlled Release. 2017;268:40-8.
61. Matijašić G, Gretić M, Kezerić K, Petanjek J, Vukelić E. Preparation of Filaments and the 3D Printing of Dronedarone HCl Tablets for Treating Cardiac Arrhythmias. AAPS PharmSciTech. 2019;20(8):310.
62. Verstraete G, Samaro A, Grymonpré W, Vanhoorne V, Van Snick B, Boone MN, et al. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. International journal of pharmaceutics. 2018;536(1):318-25.
63. Xu P, Li J, Meda A, Osei-Yeboah F, Peterson M, Repka M, et al. Development of a quantitative method to evaluate the printability of filaments for fused deposition modeling 3D printing. International journal of pharmaceutics. 2020;588:119760.
64. Lee J, Song C, Noh I, Song S, Rhee Y-S. Hot-Melt 3D Extrusion for the Fabrication of Customizable Modified-Release Solid Dosage Forms. Pharmaceutics [Internet]. 2020; 12(8).
65. Keen JM, Hughey JR, Bennett RC, Jannin V, Rosiaux Y, Marchaud D, et al. Effect of tablet structure on controlled release from supersaturating solid dispersions containing glyceryl behenate. Molecular pharmaceutics. 2015;12(1):120-6.
66. Aleksovski A, Van Bockstal P-J, Roškar R, Sovány T, Regdon G, De Beer T, et al. Comparison of metoprolol tartrate multiple-unit lipid matrix systems produced by different technologies. European Journal of Pharmaceutical Sciences. 2016;88:233-45.
67. Vervaeck A, Monteyne T, Siepmann F, Boone MN, Van Hoorebeke L, De Beer T, et al. Fatty acids for controlled release applications: A comparison between prilling and solid lipid extrusion as manufacturing techniques. European Journal of Pharmaceutics and Biopharmaceutics. 2015;97:173-84.
68. Keen JM, Foley CJ, Hughey JR, Bennett RC, Jannin V, Rosiaux Y, et al. Continuous twin screw melt granulation of glyceryl behenate: Development of controlled release tramadol hydrochloride tablets for improved safety. International journal of pharmaceutics. 2015;487(1):72-80.
69. Bhagurkar AM, Repka MA, Murthy SN. A Novel Approach for the Development of a Nanostructured Lipid Carrier Formulation by Hot-Melt Extrusion Technology. Journal of pharmaceutical sciences. 2017;106(4):1085-91.
70. Silva LAD, Almeida SL, Alonso ECP, Rocha PBR, Martins FT, Freitas LAP, et al. Preparation of a solid self-microemulsifying drug delivery system by hot-melt extrusion. International journal of pharmaceutics. 2018;541(1):1-10.
Authors
Dr Nensi Raytthatha
Corresponding Author: Dr Nensi Raytthatha
Krishna School of Pharmacy & Research
NH #8, BITS Edu Campus, Varnama, Vadodara, Gujarat 391240, India
Email: drjigarvyas@gmail.com
Telephone: +81 09428764734




Comments