- EJPPS

- Jul 6, 2022
- 13 min read
Updated: Jul 14, 2022
Peer Review Article | Open Access | Published 14th July 2022
Application of Quality by Design Approach in Development of Cefixime Trihydrate Loaded Gastro-retentive Mucoadhesive Microspheres
Priyanka Chaturvedi, Ankit Mishra, Suresh Kumar Paswan | EJPPS | 272 (2022) | https://doi.org/10.37521/ejpps.27202 Cite this article | Click to download pdf
Abstract
Cefixime is an antibiotic that belongs to the 3rd generation cephalosporin antibacterial and acts by interrupting the cell wall synthesis of bacteria. It is a weakly acidic drug primarily absorbed through the stomach and upper intestine as a unionized drug. The drug is incompletely absorbed from GIT, leading to poor bioavailability. The current research focuses on developing gastro-retentive mucoadhesive microspheres loaded with cefixime trihydrate. The drug remains in the unionized form in acidic pH, showing enhanced absorption through the stomach. Mucoadhesive microspheres of cefixime trihydrate were prepared using HPMC K15M and Carbopol 971P as carrier polymer and mucoadhesive polymer, respectively. The formulation was prepared by using the spray drying technique.
Further, the in-vitro evaluation of the mucoadhesive property of cefixime microspheres was done on the goat stomach mucosa. The study showed a strong mucoadhesion of 82% for an extended period of gastroprotection up to 6 hours. The in-vitro drug release study of microspheres was performed using 0.1 N HCl. The prepared formulation exhibited extended release for up to 8 hours. It is concluded from the above studies that the current formulation has been elicited prolonged gastric residence time as well as extended-release and provided an opportunity for better and enhanced absorption of the drug. Thus, the formulation may be projected for better therapeutic value, probably by improving the bioavailability of the experimental drugs.
Keywords: Cefixime trihydrate, mucoadhesive microsphere, gastro-retention, spray drying.
1. Introduction
Gastroretentive systems can limit extension and localize the drug in the stomach. The proximal small intestines form a few hours to enhance the gastric residence time of the desired drug. These gastro-retentive systems increase the drug residence time in the stomach, providing an opportunity for improved absorption followed by enhanced bioavailability and minimizing the wastage of the drug. The mucoadhesive polymers are widely used to enhance drug retention, which holds the drug by entrapment and resists the movement of drugs, helping to adhere to the drug formulation to the stomach and GIT mucosa. Finally, this improves the oral bioavailability of drugs by bringing the drug to proximity with the gastric mucus layer. This interaction of the bio-adhesive polymer with a mucus layer of a mucus membrane is known as mucoadhesion [1-6]. Cefixime, used as a drug molecule, is a broad-spectrum antibiotic, which prevents bacterial cell wall synthesis [7]. The drug has inadequate oral bioavailability (40-50 %) [8]. The drug suffers from a reduced half-life of 3-4 hr and is quickly eliminated from blood circulation [9]. Cefixime is very slightly soluble in water as well as acidic media. The acidic drug. The hydrated form of the drug is unionized in the stomach’s acidic environment. The unionized form of the drug has a narrow absorption window, which is mainly absorbed through the stomach [10].
The present work was intended to develop gastro-retentive mucoadhesive microspheres loaded with cefixime trihydrate. These microspheres are prepared using a spray drying process. These microspheres were optimized through the Design of Experiment (DoE) approach [11-12]. The statistical analyses were done to test and validate the independent factors and dependent response variables using response surface methodology. The response variables such as drug entrapment efficiency, particle size, and in-vitro drug release were evaluated for optimization, and studies on isolated goat stomach mucosa were also performed for the better understanding of mucoadhesive properties.
2.0 Materials and methods
Gift samples of cefixime trihydrate and Hydroxypropyl methylcellulose (HPMC K15M) were received from Schon Pharmaceuticals Ltd., Indore, and Colorcon Asia Pvt. Ltd. Verna, India, respectively. Carbopol 971P, dichloromethane, and methanol were used as purchased from SD-fine chem, India. All the ingredients used in the experimental protocol were of standardized analytical grade.
2.1 Preparation of mucoadhesive microspheres of Cefixime trihydrate
The mucoadhesive microspheres of cefixime trihydrate were prepared using the spray drying technique [13]. The carbopol 971P and HPMC K15M were chosen as mucoadhesive polymer and release controlling excipients. A weighed amount of Carbopol 971P (1000 mg) and HPMC K15M (300 mg) was dissolved in 60 ml of methanol and 40 ml of dichloromethane, respectively, and stirred separately on a magnetic field stirrer for 1 hr and mixed till the clear solution achieved. A weighed amount of cefixime trihydrate was dissolved in the above polymeric solution in the drug-polymer ratio (1:4). The resultant solution was spray-dried using the lab spray dryer (Spray mate, Jay Instruments & Systems Pvt. Ltd., Mumbai, India) to achieve drug-loaded microspheres [14]. The solution was sprayed at a 15 ml/min flow rate using a peristaltic pump at an inlet temperature of 120°C and an outlet temperature of 60°C [15].
2.2 Systematic optimization by Design of Experiment (DoE)
The response surface methodology was employed in optimizing the Box-Behnken design to investigate the effect of independent and dependent variables systematically. The 3-factors at 3-level design were used in the experiments for preparing microspheres. The independent variables were selected for the design of an experiment, such as cefixime concentration (X1), carbopol 971P (X2), and HPMC K15M concentration (X3) at three different levels (-1, 0, +1), as mentioned in table 1. The prescribed process variables were determined from the studies performed earlier, such as the solubility of the drug, polymer ratio with solvent ratio (DCM: Methanol), the viscosity of the feed, and speed of the peristaltic pump. The three independent variables (factors) considered in the preparation of cefixime trihydrate microspheres were the quantity of carbopol 971P, HPMC K15M, and cefixime trihydrate. At the same time, the particle size and cumulative % drug release till 8 hours were used as dependent variables (response variables), as shown in table 1.
Table 1: Variables and their levels in Box-Behnken Design

Table 2: Box-Behnken experimental design with measured responses

3.0 Characterization and cefixime microspheres
3.1 Determination of particle size of cefixime microspheres formulation
The particle size analysis of cefixime microspheres was performed by dispersing the microspheres in a small amount of water and analyzing them under a scanning microscope (Leica microsystems) at the magnification of 100x. The particle size of 100 microspheres was observed and analyzed for each batch. The average particle size was determined using a calibrated micrometer scale on an optical microscope.
3.2 Scanning electron microscopy.
The analyses of surface morphology of optimized microspheres were done using scanning electron microscopy (Supra 55 Zeiss). The samples were placed on aluminum stubs and stuck by carbon conductive double-faced adhesive tape (Oxon, Oxford Instruments, U.K.). A thin layer of gold coating was done using a sputtering unit before analysis at an acceleration voltage of 20 kV at different magnifications.
3.3 Differential scanning calorimetric analysis
A study of physical properties was performed by differential scanning calorimetry (Perkin Elmer 6000) analysis. Approximately weighed amount of 3 mg sample (drug- cefixime trihydrate, Polymers- HPMC K15M, carbopol 971P0, and Microsphere formulation) was placed in an aluminum pan crimped for DSC analysis. The samples were heated from 50°C to 150°C and scanned at a 20°C/min rate under nitrogen flow (20ml/min) [16].
3.4 Determination of entrapment efficiency
The entrapment efficiency of microspheres was determined by dispersing the weighed quantity of the microspheres loaded with the drug in 5 ml of methanol, sonicated the dispersion for 2 min, and the volume was made up to 100 ml with 0.1 N HCl. The solution was appropriately diluted with 0.1 N HCl concentration ranging from 0 to 100 µg/ml, and each concentration was analyzed on a UV-visible spectrophotometer (model No- 1700, Shimadzu) at 283 nm.
The entrapment efficiency of microspheres was determined using the following formula [17] in Table 2.

3.5 In-vitro drug release study
In-vitro drug release study of prepared microspheres was performed using 0.1N HCl as drug-releasing media. The required amounts of drug-loaded microsphere were added to a beaker containing 200 ml of 0.1N HCl of the drug release media and stirred at 100 RPM. At a predetermined time point (5 ml aliquot of drug samples were withdrawn, centrifuged for 5 min at 11000 rpm (Eppendorf cooling centrifuge) filtered by 0.2-micron membrane filter analyzed by UV-visible spectrophotometer at 283 nm. The sample withdrawn was replaced by the same amount of fresh drug media. The experiments were carried out in triplicates, and average values obtained were recorded [18].
3.6 In-vitro mucoadhesion study
The mucoadhesive property of experimentally optimized and prepared microspheres in batch-wise was evaluated on a strip of goat stomach mucous membranes (3x1cm) procured from the local slaughterhouse in Indore. Stomach mucous membranes were removed and cleaned using normal saline solution. The membrane was attached to a glass slide, and an accurately weighed amount of microspheres (50 mg) were spread uniformly over the surface of the intestinal mucosa [19]. The mucosal surface was rinsed with phosphate buffer (6.8) using the syringe pump at a 1 ml/minute flow rate. Washings were collected, centrifuged (Eppendorf company, minispin) for 7000 RPM for 15 minutes, and dried. The membrane was attached to a glass slide, and accurately weighed microspheres (50 mg) were spread uniformly on the intestinal mucosa surface [3, 20].

Where,
Wa = weight of microspheres applied
WL = weight of microspheres leached out
4.0 Results and Discussion
4.1 Formulation and optimization of cefixime microspheres by response surface methodology
The results obtained from the optimized formulation were statistically analyzed for response variables by using Design Expert 7.1.6 (trial version) software (Stat-Ease Inc., Minneapolis, USA). The software proposed a total of 15 experiments according to box banking design. Models were selected based on sequential comparison and lack of fit test. The significance of the models was further confirmed by statistical analysis. The design was evaluated using statistical analysis by the sum of squares, R-squared, and p-value. The above tool inferred that in-vitro release followed the quadratic and mean model, and drug content followed the 2FI model. The statistical summary of response variables is shown in Tables 3 and 4. The following polynomial equations in terms of essential factors were generated to demonstrate the relationship between the formulation variable.
Table 3: Summary of results of (a) Sum of squares, (b) lack of fit, and (c) R-square analysis for measured responses

Table 4: Summary of results of (a) Sum of squares, (b) lack of fit, and (c) R-square analysis for measured responses


Fig.1:Three dimensional response surface plot showing (A) the effect of cefixime trihydrate and carbopol 971 P concentration on particle size, (B) the effect of HPMC K15M and carbopol 971P concentration on particle size.

Fig.2: Three-dimensional response surface plot showing (A) the effect of HPMC K15M and concentration on % release in 15 minutes, (B) the effect of cefixime trihydrate and carbopol 971P concentration on % release in 15 minutes.
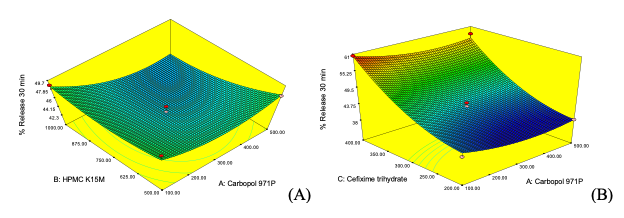
Fig.3: Three-dimensional response surface plot showing (A) the effect of Carbopol 971P and HPMCK15M concentration on % release in 30 minutes, (B) the effect of cefixime trihydrate and Carbopol 971P concentration on % release in 30 minutes.

Fig.4: Three-dimensional response surface plot showing (A) the effect of Carbopol 971P and HPMCK15M concentration on % release in 60 minutes, (B) the effect of cefixime trihydrate and Carbopol 971P concentration on % release in 60 minutes.

Fig.5: Three-dimensional response surface plot showing (A) The effect of Carbopol 971P and HPMCK15M concentration on % release in 480 minutes, (B) The effect of cefixime trihydrate and carbopol 971P concentration on % release in 480 minutes.


Fig.6: Cumulative % drug release v/s time plot of cefixime trihydrate mucoadhesive microspheres optimization batches
4.2 Prediction of optimized cefixime trihydrate mucoadhesive microspheres formulation
Statistical analysis of the data was obtained using design expert software, keeping the constraints and criteria on the desired characteristics of the final formulation of optimization batches,’ i.e., minimum particle size and required sustained release drug release pattern as shown in table 1. The software predicted the formulations with desirability close to 1. The formulation with maximum desirability of 0.902 was selected as the best predictor variable for designing and preparing an optimized formulation. The desirability contour and response surface plots predicted the formulation with maximum desirability and cumulative % drug release of optimized batch.

Fig.7: Three dimension plot showing the microsphere formulation of maximum desirability

Fig.7: Three dimension plot showing the microsphere formulation of maximum desirability
Table 5: Predicted and optimized variables of cefixime trihydrate mucoadhesive microsphere formulation


Fig. 9: Release profile of predicted and observed formulation of cefixime trihydrate mucoadhesive microspheres

Fig. 10: Linear plots between observed and predicted values of % cumulative drug release
4.3 In-vitro characterization
During the optimization process of microspheres, the entrapment efficiency does not change much more in the spray drying process. The maximum entrapment efficiency was found to be 92%. The mean particle size of spray-dried microspheres was determined by optical microscopy; their size ranges from 4.5 -5.3 µm. The scanning electron micrograph of cefixime trihydrate mucoadhesive microspheres is shown in fig.11. Microspheres obtained from the observation were of uniform size with a smooth surface.
The differential scanning colorimetric patterns of the microspheres are shown in Fig. 12. The DSC thermograph shows the endothermic peak of cefixime trihydrate at 117°C. No endothermic peaks were observed in DSC graphs of excipients, specially Carbopol 971 and HPMC K15M. The absence of any specific peak at 117°C in microsphere formulation confirmed the conversion of the physical form of cefixime trihydrate from the crystalline peak into an amorphous form. Observations of the DSC studies confirmed the formation of a solid dispersion of cefixime trihydrate in microspheres.

Fig.11. Scanning electron microphotographs of optimized microspheres

Fig.12. Overlay graph of DSC analysis
4.4 In-vitro drug release study by direct addition of microsphere in to release media
In-vitro drug release study by direct addition of microsphere into release media In-vitro drug release study of optimized microspheres by direct addition of microsphere was performed by adding microsphere to release media held in a beaker and stirred with the help of a magnetic stirrer. The required amounts of drug-loaded microspheres were added to a beaker containing 200 ml of dissolution media and were kept in a magnetic stirrer at 100 RPM. At a predetermined time point, a 5 ml aliquot of drug sample was withdrawn, centrifuged for 5 min at 11000 rpm (Eppendorf cooling centrifuge) filtered by 0.45-micron membrane filter, and drug entrapped in a microscope was quantified by U.V.- visible spectrophotometer at 283 nm. The withdrawn samples were replaced by the same amount of fresh medium a predetermined period performed in triplicates [21].
About 30% of cefixime trihydrate was released in the initial 15 minutes, showing a burst effect. The drug adsorbed on the surface of microspheres may be attributed to burst release. This initial burst effect was beneficial to achieve effective plasma concentration after administering cefixime trihydrate mucoadhesive microsphere. The remaining drug was entrapped within the microspheres released consistently throughout 6 hrs. The results for in-vitro release studies are reported in table 2 and graphically represented in Fig. 6 and Fig.9.
Optimization of the developed formulation was done by Box-Behnken design using Design Expert 7.1.6 (trial version) version software (Stat-Ease Inc., Minneapolis, USA). The cefixime trihydrate-loaded gastro retentive microspheres were prepared using a spray dryer. The concentration of cefixime trihydrate, carbopol 971P, and HPMC K15M was selected as independent variables, particle size distribution, and percent cumulative drug release at 15, 30, 60, 120, 240 360, and 480 minutes were selected as response variables for optimization studies. The results obtained from the experiments were statistically analyzed for response variables. Response surface graphs, contour plots, and 3D contour plots were generated and analyzed for each response variable. The in-vitro percentage drug release and in-vitro mucoadhesive studies indicated that the prepared formulation possesses both release and mucoadhesion properties. The mucoadhesive microspheres of Cefixime trihydrate using the predicted optimized formulas were prepared and experimentally validated. The in-vitro drug release study of the optimized batch showed a consistent drug release of the drug up to 8 hr with mucoadhesion of 82% up to 6 hr.
4.5 Evaluation of mucoadhesion of microspheres
The in-vitro mucoadhesive properties of the optimized batches of microspheres were found to be 82% after six hours of microsphere application. The percentage of mucoadhesion was notably increased with the incorporation of carbopol 971P in the microspheres, which indicated that carbopol 971P has a strong ability to interact with mucus. The more carbopol 971P was incorporated, the better the retention effect.
5. Conclusion
The oral administration of cefixime trihydrate has poor absorption, reduced bioavailability, and a short half-life. In order to overcome these drawbacks, the controlled release gastro-retentive microspheres were developed. The prepared gastro retentive microspheres are likely to be retained in the gastric area by adhering to the mucosal membrane for a more extended period, as supported by in vitro studies after developing the current drug into microspheres. Hence, from the above results and findings, it is concluded that the developed gastro retentive mucoadhesive microsphere can be used as a potential drug delivery system after preclinical and clinical trials, which are expected to provide antibacterial activity with longer residence time in the gastric region by adhering into gastric mucosa. Further, it needs in vivo gastro retentive and clinical studies to evaluate its applicability at the actual performance of the developed cefixime trihydrate microsphere formulation at clinical practice.
Conflict of Interest - There is no conflict of interest.
References
01. Zhao S., Lv Y., Zhang J.B., Wang B., Lv G.J., Ma X.J., Gastroretentive drug delivery systems for the treatment of Helicobacter pylori, World J Gastroenterol (2014); 20(28): 9321-9.
02. Pund S., Joshi A, Vasu K., Nivsarkar M., Shishoo C., Gastroretentive delivery of rifampicin: in vitro mucoadhesion and in vivo gamma scintigraphy, Int J Pharm 2011; 411(1-2): 106-12.
03. Liu Y,, Zhang J., Gao Y., Zhu J., Preparation and evaluation of glyceryl monooleate-coated hollow-bioadhesive microspheres for gastroretentive drug delivery, Int J Pharm. 2011; 413(1-2):103-9.
04. Kyada C.,. Ranch K., Shah D., Optimization of mucoadhesive microspheres of acyclovir by applying 32 full factorial design, J Drug Del Sci Tech. 2014; 24(1): 61-68.
05. Vasir J.K., Tambwekar K., Garg S., Bioadhesive microspheres as a controlled drug delivery system, Int J Pharm. 2003; 255(1-2):13-32.
06. Lehr C.M., Bouwstra J.A., Schacht E.H.,. Junginger H.E, In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers, Int J Pharm. 1992; 78(1-3):43-48.
07. Tan B.J.K, Cefixime use in children: When and why, Can J Infect Dis. 1995; 6(4):204-205.
08. Paul Y., M. Kumar, Singh B., Formulation and in vitro evaluation of gastroretentive drug delivery system of cefixime trihydrate, Int J Drug Dev & Res. (2011); 3(4): 148-161.
09. Kandhro A.A., Laghari A.H., Mahesar S.A., Saleem R., Nelofar A., Khan S.T., Sherazi S.T.H., Application of attenuated total reflectance Fourier transform infrared spectroscopy for determination of cefixime in oral pharmaceutical formulations, Spectrochim Acta A Mol Biomol Spectrosc. (2013); 115: 51-56.
10. Maestrelli F.,Jug M., Cirri M., Kosalec I., Mura P., Characterization and microbiological evaluation of chitosan-alginate microspheres for cefixime vaginal administration, Carbohydr Polym (2018); 192:176-183.
11. Hooda A., Nanda A., Jain M., Kumar V., Rathee P., Optimization and evaluation of gastroretentive ranitidine HCl microspheres by using design expert software, Int J Biol Macromol. 2012; 51(5): 691-700.
12. Karnachi A.A., Khan M.A., Box-behnken design for the optimization of formulation variables of indomethacin coprecipitates with polymer mixtures, Int J Pharm 1996;131(1): 9-17.
13. Rosemary MJ. Mucoadhesive microspheres of ferrous sulphate–A novel approach for oral iron delivery in treating anemia. Colloids and Surfaces B: Biointerfaces. 2020 Nov 1;195:111247.
14. Harikarnpakdee S., Lipipun V., Sutanthavibul N., Ritthidej G.C., Spray-dried mucoadhesive microspheres: preparation and transport through nasal cell monolayer, AAPS Pharm Sci Tech. 2006;7(1):E12-E12.
15. Baek J.S., Yeon W.G., Lee C.A, Hwang S.J., Park J.S., Kim D.C., Cho C.W., Preparation and characterization of mucoadhesive enteric-coating ginsenoside-loaded microparticles, Arch Pharm Res. 2014; 38.
16. Hou J.Y., Gao L.N., Meng F.Y., Cui Y.L., Mucoadhesive microparticles for gastroretentive delivery: preparation, biodistribution and targeting evaluation, Mar Drugs. 2014;12(12): 5764-87.
17. Nappinnai M., Sivaneswari S., Formulation optimization and characterization of gastroretentive cefpodoxime proxetil mucoadhesive microspheres using 32 factorial design, J Pharm Res.2013; 7(4): 304-309.
18. Wong T.W., Chan L.W., Lee H.Y., Heng P.W.S., Release characteristics of pectin microspheres prepared by an emulsification technique, J Microencapsul 2002; 19(4) : 511-522.
19. Gaba P., Singh S., Gaba M., Gupta G.D., Galactomannan gum coated mucoadhesive microspheres of glipizide for treatment of type 2 diabetes mellitus: In vitro and in vivo evaluation, Saudi Pharm J. 2011;19(3): 143-52.
20. Dandagi P.M., Mastiholimath V.S., Gadad A.P., Iliger S., Mucoadhesive microspheres of propanolol hydrochloride for nasal delivery, Indian J Pharm Sci. 2007; 69.
21. Tao Y., Lu Y., Sun Y., Gu B., Lu W., Pan J., Development of mucoadhesive microspheres of acyclovir with enhanced bioavailability, Int J Pharm. 2009; 378(1): 30-36.
Author Information
Priyanka Chaturvedi a,b, Ankit Mishra* c, Suresh Kumar Paswanb
a. Department of Pharmaceutics, Sagar Institute of Research and Technology – Pharmacy, Ayodhya bypass, Bhopal, MP, India. 462003
b. Department of Pharmacy, Shri G. S. Institute of Technology & Science, Indore, MP, India. 452007
c. Department of Pharmaceutics, Faculty of Pharmacy, VNS Group of Institutions, Neelbud, Bhopal, MP, India. 462044
Corresponding Author -
Dr Ankit Mishra
ORCID - 0000-0003-0795-8417
Associate Professor
Department of Pharmaceutics,
Faculty of Pharmacy,
VNS Group of Institutions,
Neelbud, Bhopal, MP, India
Pin-462044
Email: mishraaa@gmail.com
Telephone: +919827930533
Other Information –
Priyanka Chaturvedi
ORCID – 0000-0003-3780-7097
Acknowledgment
The authors would like to acknowledge Shri G. S. Institute of Technology & Science for providing the research facility.



Comments