- EJPPS

- Jan 9, 2021
- 14 min read
Peer Review Article | Open Access | Published 9th January 2021
Effective Re-usable Cleanroom Garments and Evaluation of Garment Life
T Eaton AstraZeneca, Macclesfield, & W Whyte James Watt Building South, University of Glasgow, UK | EJPPS | 254 (2020) | Cite this article https://doi.org/10.37521/ejpps.25401 | Click to download pdf
Summary
Cleanroom garments are used to control the airborne dispersion of contamination from people into the cleanroom. The effectiveness of the garment in controlling the dispersion of contamination is a function of the fabric and design of garments, and test methods used to ascertain the effectiveness of garments are discussed in this article. These test methods can be used when choosing garments for use in a cleanroom but were used in this article to determine the deterioration of garments through use. Cleanroom garments were subjected to increasing numbers of decontamination cycles, which included sterilisation by gamma radiation, up to a maximum of 70. At defined number of decontamination cycles, the garment’s fabric was compared to a new fabric by visual examination, by a scanning electron microscope, and by physical tests of key performance parameters. It was concluded that the performance of the fabric remained acceptable up to 50 decontamination cycles. This conclusion was supported by the low dispersion rate of particles and microbe-carrying particles in a dispersal chamber from personnel wearing the garments. After 50 decontamination cycles, a low dispersion rate of 0.2/s of microbe-carrying particles from personnel wearing the garments was obtained and a 194-fold reduction in the microbial dispersion rate compared to cleanroom undergarments.
Key words: cleanroom garments, garment life, contamination controlKeywords
1. Introduction
Cleanroom garments are a key contamination control method used to limit the transfer of particles from personnel into the surrounding environment. A proportion of these particles are microbe carrying particles (MCPs), and for pharmaceutical and healthcare cleanrooms, they present a risk that must be managed. Garments may be single use or reusable and both have the same contamination control requirements. Single use garments are typically used in situations where contamination can occur from harmful biological, chemical, or radioactive substances, or where low numbers of cleanroom garments are required. Following a single use, they are simply disposed. Re-usable garments are used many times, and between uses are subjected to decontamination cycles, which normally consist of controlled washing, drying, packaging and where appropriate, sterilisation. These decontamination cycles should not significantly reduce the contamination control properties of the garment through its life.
Whyte and Bailey ¹ ² developed tests for assessing the contamination control properties of cleanroom garments and drew attention to the deterioration of garments during use. They also noted that heavily calandered garments lose their effectiveness much more quickly than lightly calandered garments. Ljungqvist and Reinmuller ³, and Romano et al ⁴, used a dispersal chamber to measure the dispersion rate of airborne contamination from personnel wearing clothing that had been subjected to different numbers of decontamination cycles. They found that the effectiveness of garments reduced during use but was acceptable up to about 50 decontamination cycles. Ljungqvist and Reinmuller ⁵ further investigated garments using a combination of fabric test methods and dispersal chamber results and confirmed their previous conclusions.
Different fabrics used to manufacture cleanroom garments deteriorate at different rates and we were interested in studying a fabric that had not been previously investigated but appeared to be very effective in reducing the dispersion of airborne contamination. We also wished to study the deterioration of a fabric with tests that included appearance to the eye and use of an electron microscope, as well as studying the deterioration of fabrics over time in more detail.
2. Requirements for cleanroom garments
Information relating to selection, specification, maintenance, and testing of garments for use in various types of cleanrooms is readily available ⁶ ⁷. For pharmaceutical and healthcare cleanroom applications, there are a number of essential and desirable requirements that need to be considered, and these are summarised in Table 1. Other parameters, such as flame retardancy, chemical resistance, waterproofness, water repellency, and anti-microbial surface properties, may be required for certain applications. The requirements that the authors consider to be the most important are included in Table 1.
Table 1 Requirements for effective cleanroom garments

3. Considerations of choice of fabric for re-usable garments
For re-usable garments, the requirements for garment fabrics that are summarised in Table 1 are discussed in this section. The design and effectiveness of garments are discussed in Section 4.
3.1 Garment fabric
A key consideration for effective contamination control is the garment fabric. Popular everyday fabrics are made from either cotton or a mixture of polyester and cotton (polycotton). Polycotton fabrics are woven from yarns made by twisting together the short staple fibres of cotton with the continuous fibres of polyester to form a cohesive yarn. Shown in Figure 1 is the magnified (x 50) image of a polycotton fabric. It can be seen why this fabric, as well as cotton fabrics are not suitable for cleanroom applications as the fibre ends protrude from these yarns and are constantly broken off during normal wear and both fibres and small particles are continually shed into the cleanroom.

Figure 1 Polycotton fabric showing protruding fibre ends (x 50 magnification).
The solution to the problem of the particle and fibre shedding by cotton and polycotton fabrics is to use monofilament plastic thread to produce the yarn. The continuous nature of this yarn ensures that fibre and particle shedding is greatly reduced. The most commonly re-usable woven fabrics used in cleanrooms are currently made from 100% monofilament polyester. Shown in Figure 2 is the integral and continuous nature of this type of fabric.

Figure 2 100% monofilament polyester fabric (x 50 magnification).
Pores occur in fabrics where the yarns cross and these pores determine the rate at which particles, air, and water vapour, pass through. To minimise the transfer of airborne contaminants from wearer to cleanroom, the fabric must be tightly and reliably woven to produce a small pore size of a consistent size. The tightness of the weave of cotton or polycotton fabric is normally inadequate to control the dispersion of skin and clothing particles from the wearer, including those that carry microbes, as they will easily pass through the space where the yarns cross. However, it is not only cotton and polycotton fabrics that suffer from this problem as fabrics made from monofilament fibres can also be ineffective. Shown in Figure 3 is a fabric woven from monofilament thread which has large pores with an equivalent pore diameter of about 100 µm. This fabric is ineffective in reducing the dispersion of particles and MCPs from personnel as they can easily pass through it. Garments used in cleanrooms should, therefore, be manufactured from a fabric that is tightly woven from monofilament thread.

Figure 3 Monofilament fabric showing large pores with an equivalent particle diameter of approximately 100 µm.
3.2 Fabric test methods
To assess the likely performance of a reusable fabric in a cleanroom, it is necessary to test a range of its properties. It is common to find that the fabric’s properties are provided by the manufacturer but several of these properties may not be directly relevant to the contamination control needs of a pharmaceutical or healthcare cleanrooms. No ISO standard exists that details the relevant properties required for a cleanroom fabric but IEST–RP-003.4 ⁶ includes contamination control property tests developed by Whyte and Bailey ¹,² for use in cleanrooms. Test methods also exist as national standards that cover individual properties of fabrics and these are often combined, although different fabric manufacturers use different tests to describe their fabrics. It is, therefore, important to ensure the fabrics have been subject to relevant test methods, and the tests that the authors consider the most important are given in Table 2.
Table 2 Fabric test methods

4. Fabric investigated
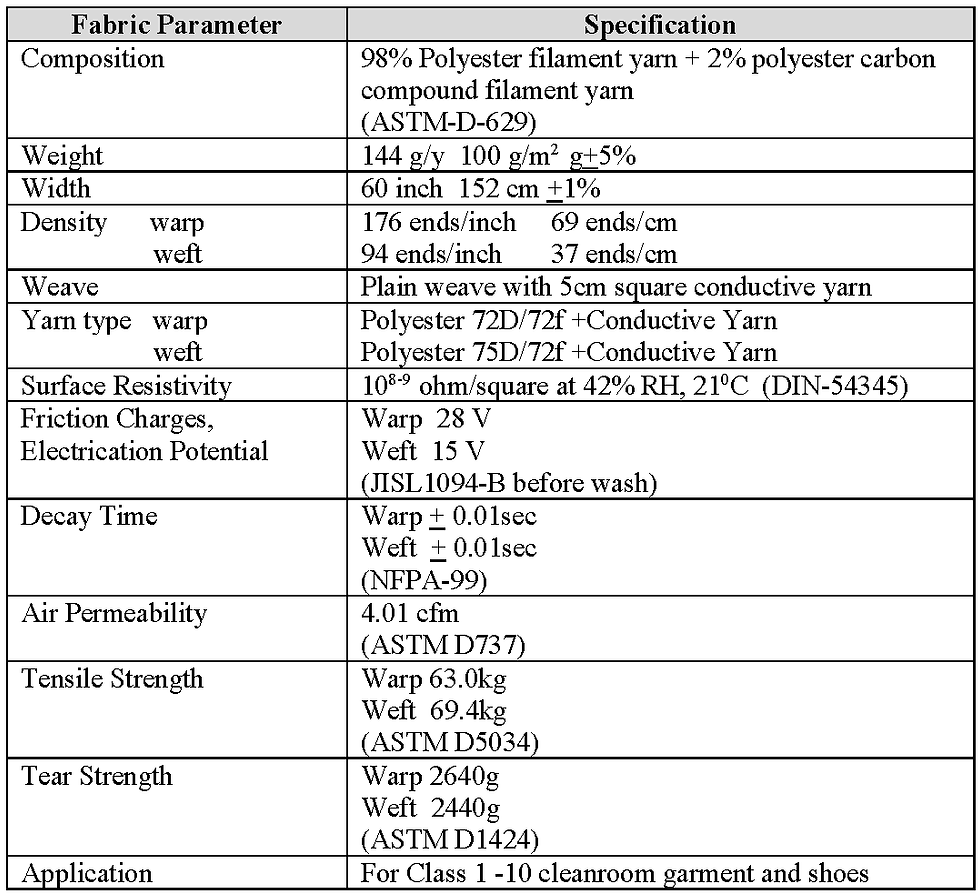
The manufacturer’s specification of the monofilament polyester fabric that was investigated is shown in Table 3 and, where applicable, the test method is included in parenthesis. The fabric investigated was a JG type (WF5505-JG) supplied by Asiatic Fiber Corporation and is widely utilised for garments in the pharmaceutical industry. However, without a detailed knowledge of fabrics and the test methods used, it is difficult to understand from the information given in Table 3 how well the fabric would perform in a pharmaceutical or healthcare cleanroom to the required parameters shown previously in Table 2.
Table 3 Specification for mono filament polyester fabric JG (WF5505-JG)
5. Assessment of fabric performance
The fabric specification shown in Table 3 relates to new garments that have not been subjected to any decontamination cycles. For re-usable garments, it is important to determine the condition of the fabric after a number of decontamination cycles, in order to define an appropriate garment lifetime.
To assess the condition of the fabric following decontamination, garments fabricated from JG (WF5505-JG) material were subjected to a number of accelerated standard decontamination cycles (wash, dry, primary pack and gamma radiation sterilisation at 25 kGy) completed by a specialised cleanroom garment laundry company. Fabric from garments that had completed 10, 25, 50 and 70 cycles were tested and compared with new (no decontamination cycles) garment fabric by an independent specialist testing company, using the following test methods, to evaluate key contamination control parameters.
Visual appearance
Equivalent pore diameter (IEST-RP-CC003.4. 2011 ⁶)
Particle removal efficiency (In-house test based on IEST-RP-CC003.4. 2011 method ⁶)
Dry linting propensity (ISO 9073-10 ⁸)
Scanning electron microscopy (SEM) imaging to determine any change to the fabric structure.
6. Results
The results of the testing are shown in Figure 4 to Figure 8 for visual appearance, equivalent pore diameter, particle removal efficiency, dry linting propensity, and SEM imaging parameters, respectively.

Figure 4 Visual appearance of garment fabric after defined number of decontamination cycles

Figure 5 Fabric equivalent pore diameter after defined number of cycles
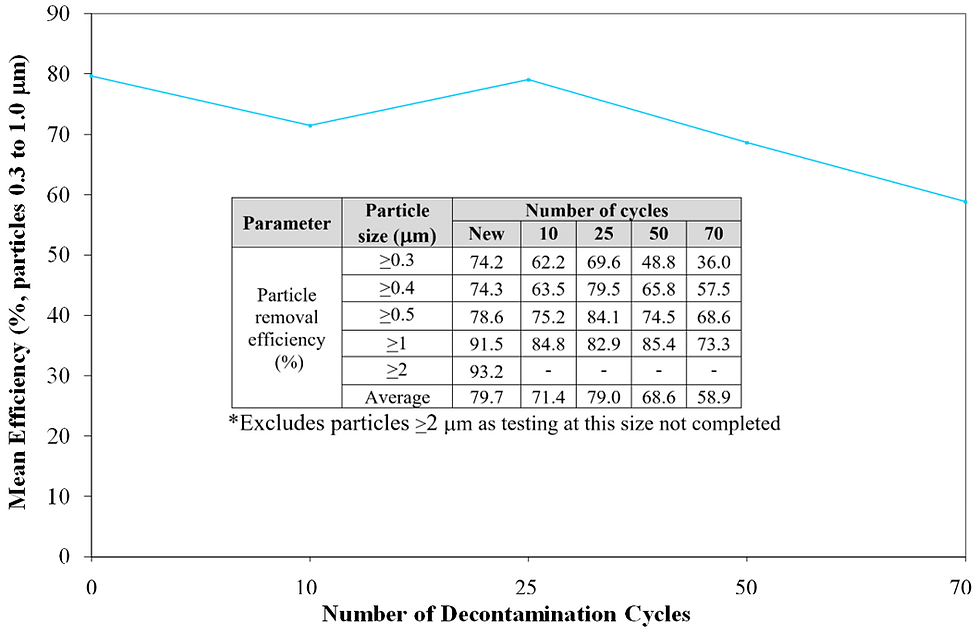
Figure 6 Average particle removal efficiency of particles ≥0.3µm, ≥0.4 µm, ≥0.5 µm, ≥1 µm after defined number of decontamination cycles.

Figure 7 Fabric dry linting propensity, particles ≥0.5 µm after defined number of cycles.

Figure 8 New fabric (left) and fabric that has been subjected to 50 decontamination cycles (right) Magnification of upper photographs x 200 and lower photographs x 500
7. Determination of overall garment effectiveness
7.1 Design of garment studied
The garment studied in the dispersal chamber to find the dispersion rate of MCPs from personnel was as follows. The garment was produced from the fabric being studied and designed to ensured that all skin surfaces of the wearer were covered. It consisted of boots, gloves, hood, and mask and is typical of garments used in an EU GGMP ⁹ Grade B cleanroom. It is shown in Figure 9. The fabric edges were sealed before being sown together to ensure no fibre break-out within the enclosed seams. The number of seams were minimised and totally enclosed using a double stitching technique to encapsulate all cut and sealed edges. To enhance operator comfort and ease of donning, stud fastenings for personal adjustments around the wrist, neck, and hood area’s were avoided and elasticated wrists and quick adjust ladder lock fastenings utilised. The coverall fastening was achieved with a tight fitting (spiral design) polyester zipper which was further protected with a material flap (placket) to provide additional containment.

Figure 9 Cleanroom garment and associated attire studied
7.2 Test method
The total contamination control effectiveness of the design of a garment and its fabric can be determined by use of a dispersal chamber. A dispersal chamber was first described by Whyte, who used a chamber supplied with a known quantity of filtered air to ascertain the dispersion rate of MCPs from people wearing different clothing ¹⁰.
The dispersal chamber used in these experiments is shown in Figure 10. It is 0.7m x 0.5m x 2m high and made of a metal frame with glass sides and a variable speed fan which supplies particle free air from a HEPA filter at the top of the chamber. The volume of particle free air that is supplied to the chamber is just over 700 L/min, and is balanced by the removal of air by a high-volume bacterial sampler (Casella slit sampler) operating at
700 L/min and connected to the base of the chamber via a sampling duct. The Casella sampler had recently been calibrated and had an air velocity through its slits of 66 m/s and d50 of 0.8 µm. A calibrated particle counter (Lasair-310) operating at 28.3 L/min was connected to a separate sampling port that is also located at the base of the chamber. The rate at which airborne particles and MCPs are dispersed from personnel is therefore measured. The detailed operation of such a chamber is discussed elsewhere ¹¹.

Figure 10 Dispersal chamber used for testing garments
Testing was carried out on 3 people (2 males, 1 female). Each subject was tested as they marched on the spot and moved their arms up to their shoulders at a rate of 1 per second. This was carried out while wearing both cleanroom undergarments, and cleanroom garments on top of the undergarments. The subjects exercised for 1 minute until the airborne contamination reached a steady state and continued to exercise during air sampling. The air sampling was carried out for 1 or 5 minutes, depending upon expected dispersion rates. There was a 5-minute interval between each test to ensure an adequate clean up period.
The undergarments consisted of a short-sleeved top with separate trousers made from a polyester and cotton mix and are specialist pharmaceutical undergarments suitable for wearing beneath cleanroom garments and complemented by mop cap and plant shoes. The cleanroom garments and complementary items were those discussed in the previous sections of this article, and all had completed 50 decontamination cycles.
The air sampler utilised plates containing tryptone soya agar, supplemented with 0.5% polysorbate 80. Polysorbate 80 is commonly used to neutralise disinfectants in cleanrooms but this was not the purpose in this situation. It was used to provide fatty acids (oleic acid) as a source of nutrition to aid the growth of lipophilic skin bacteria. All plates were incubated before use and checked for sterility. After use, the plates were incubated aerobically at 32.5°C (± 1.5°C) for 3 days and examined for microbial growth. Due to the determined combined losses from the Casella air sampler and the air intake duct, the resultant counts were multiplied by a factor of 2.6 to take this into account ¹¹. The particle counter simultaneously recorded the concentrations of the total particles ≥0.5 µm and ≥5 µm per m³ during the exercising. With knowledge of the air supply rate to the dispersal chamber and the sampling rate of the air samplers the dispersion rate was obtained.
7.3 Results from dispersal chamber
Shown in Tables 4 and 5, respectively, are the dispersion rates of particles ≥0.5 µm and ≥5 µm and MCPs for the subjects when wearing undergarments and when wearing cleanroom garments on top of undergarments. These tables also include the average dispersion rates and the number of times reductions when comparing cleanroom garments to undergarments. Dispersal rates are normally reported as number per second, but as it is easier to comprehend dispersal rates per minute, these are the units utilised in Tables 4 and 5. All the results are rounded to whole numbers and are shown graphically in Figure 11.
Table 4 Dispersion rates per minute, particles ≥0.5 µm and ≥5 µm, when wearing undergarments and cleanroom garments
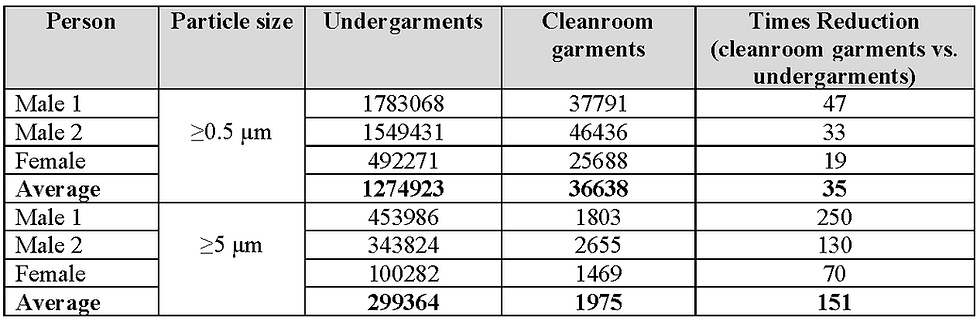
Table 5 Dispersion rates per minute, MCPs, when wearing undergarments and cleanroom garments


Figure 11 Dispersion rates of MCPs and particles from 3 people wearing cleanroom undergarments and cleanroom garments
8. Discussion and conclusions
Information is provided in this article about tests used to determine the contamination control properties of fabrics used to manufacture garments worn in pharmaceutical and healthcare cleanrooms. These tests can be used when garments are first selected for use in the cleanroom but in this article, they were used to investigate the deterioration of new garments. Tests were carried out on a previously unstudied fabric when new, and after 10, 20, 50, and 70 decontamination cycles. These cycles included washing, drying, sterilisation by gamma radiation. It is probable that sterilisation by autoclaving would have given different results, but this was not investigated.
The appearance of the fabric was observed as the number of decontamination cycles increased from new to 70 cycles and there was a clear loss in the fabric colour, as shown in Figure 4. In addition, the fabric was noticeably thinner after 70 cycles and it was difficult to put on garments without tearing the fabric. This change in the fabric was thought to indicate deterioration in the contamination control properties of the fabric and was investigated.
The weave of the fabric was observed by a scanning electron microscope and images are shown in Figure 8 of the new fabric and after 50 decontamination cycles. After 50 cycles, the fabric was shown to maintain a tight and consistent weave, with no indication of material breakdown, including the integral carbon encapsulated grid, and there was little or no difference from the new fabric. However, although no image is included in this article, it was found that after 70 cycles, there was a break-up of the carbon encapsulated grid.
As reported in the graph in Figure 5, the equivalent pore diameter of the new fabric was 11.3 µm and a reasonably consistent profile of pore size was maintained throughout the increasing number of decontamination cycles. After 70 cycles the pore size was the same as the new fabric. Pore size is a key parameter used to predict the ability of a woven fabric to provide effective barrier and containment control, and the smaller the pore size the more effective the control. It was expected that the particle removal efficiency would maintain a similar consistent profile through decontamination cycles. However, this was only partly confirmed by the results given in the table in Figure 6, as the overall drop in particle removal efficiency after 50 cycles was found to be 13.9%, and after 70 cycles it was 26.1%.
Tests were also carried out on the release of particles from the fabric (dry linting propensity). The results are given in the graph in Figure 7 and they showed an increase in particles after 10 contamination cycles but all subsequent results up to 70 cycles were less than those recorded for the new fabric.
When taking into account all of the above information, it is considered that a limit of 50 decontamination cycles should be placed on the use of the fabric studied before replacement. To confirm this, and to study the dispersion rates of MCPs from garments worn by personnel and made from this fabric, a dispersal chamber was used. Only one set of results was obtained from the three personnel who participated but the dispersal profiles from each individual were consistent. The control of dispersion of particles is related to the pore size of the garment’s fabric and, therefore, the larger the particle, the more effective the fabric will be. This was confirmed by the reductions of particles ≥0.5 µm, ≥5 µm, and MCPs (average size typically 12 µm ¹¹ ¹²), which gave average reductions compared to cleanroom undergarments of 35, 151 and 195-fold, respectively. It was also found that the average dispersion rate of MCPs from the three personnel when wearing garments that had gone through 50 decontamination cycles was 10/minute (0.2/s). This was close to the lower dispersion rate from personnel wearing garments made from different fabrics that had gone through 50 decontamination cycles and reported by Ljungqvist and Reinmuller ⁴ to have satisfactory emission rates of 9.8/s, 1.9/s, 0.1/s and 0.2/s.
Using the information from the contamination control tests of the fabric studied and the dispersion rate in the chamber, it appears that the control of the dispersion of MCPs by the fabric and garments was satisfactory up to 50 decontamination cycles but not 70. However, it has been shown that different fabrics will give different rates of change of their contamination control performances over time ², and it is also likely that the type of decontamination cycle will affect the rate of deterioration of fabrics. It may, therefore, be considered appropriate to investigate garments when first introduced into a cleanroom, and over time, by the use of tests described in this article to determine how many decontamination cycles can be used before garments lose their contamination control effectiveness.
References
01. Whyte W and Bailey PV. Reduction of microbial dispersion by clothing. Journal of Parenteral Science and Technology 1985; 39(1): 51-60.
02. Whyte W and Bailey PV. Particle dispersion in relation to clothing. The Journal of Environmental Sciences 1989, March/April: 43-49.
03. Ljungqvist B and Reinmuller B. People as a contamination source: cleanroom clothing systems after 1, 25 and 50 washing /sterility cycles. European Journal of Parenteral and Pharmaceutical Sciences 2003; 8(3): 75-80.
04. Romano F, Ljungqvist B, Reinmuller B, Gusten J and Joppolo CM. Performance test of technical cleanroom clothing systems Proceedings of Indoor Air 2016, 14th International Conference on Indoor Air Quality and Climate, Ghent, Belgium, 2016.
05. Ljungqvist B and Reinmüller B. People as a contamination source –dispersal chamber evaluation of clothing systems for cleanroom and ultra clean operation rooms. Report number D2014:01, Chalmers University of Technology, Sweden, 2014.
06. IEST-RP-CC003.4. Garment system considerations for cleanrooms and other controlled environments. Institute of Environmental Sciences and Technology. 2011
07. Clayton N and Eaton T. The Micronclean big blue cleanroom handbook. 2011. ISBN 9780-9570735-0-0.
08. ISO 9073-10:2003. Textiles. Test methods for nonwovens. Lint and other particles generation in the dry state. International Organization for Standardization, Geneva, Switzerland.
09. EU GGMP (2008). The rules governing medicinal products in the European Union –Volume 4 -EU guidelines to good manufacturing practice – medicinal products for human and veterinary use – Annex 1 –Manufacture of sterile medicinal products. European Commission, Brussels.
10. Whyte W, Vesley D and Hodgson R. Bacterial dispersion in relation to operating clothing. Journal of Hygiene 1976; 76: 367-378.
11. Whyte W and Hejab M. Particle and microbial airborne dispersion from people. European Journal of Parenteral and Pharmaceutical Sciences 2007; 12(2): 39-46.
12. Noble WC, Lidwell OM and Kingston D. The size distribution of airborne particles carrying micro-organisms. Journal of Hygiene 1963; 61:385–391.
Author Information
Corresponding Author: Tim Eaton, Sterile Manufacturing Specialist
AstraZeneca,
UK Operations,
Silk Road Business Park,
Macclesfield
Cheshire. SK10 2NA
England
Email: tim.eaton@astrazeneca.com
Telephone: +44(0) 1625 514916


Comments