- lauraclark849
- Dec 9, 2025
- 23 min read
Updated: Dec 18, 2025
Technical Review Article | Open Access | Published 18th December 2025
Microemulgel - A promising novel formulation for topical drug delivery
Gupta Ashish¹, Darwhekar Gajanan¹, Jain Sachin Kumar² | EJPPS | 304 (2025) https://doi.org/10.37521/ejpps30406
Back to Journals | Article |Abstract| References | Authors
Abstract
Microemulgel is a new and promising drug delivery system employed in the pharmaceutical industry. It is a stable dispersion consisting of an oil, surfactant, and clear aqueous phase that can be stable either thermodynamically or kinetically. The usage of microemulgel as a new transdermal drug delivery technique has grown because of its advantages over its existing oral and topical drug delivery methods, including its ability to prevent poor drug bioavailability and pharmacokinetic variability.
The novel technique developed by adding micro-emulsion with gel improves stability and permits fast, controlled drug release. The microemulgel drug delivery system is a formulation-related technology designed to enhance drug absorption and lipophilic drug therapeutic profile. A significant growth in microemulgel has been noted in recent years owing to the high toleration of the formulation by patients and the preparation’s enhanced patient acceptance since it is non-greasy, easily applied with convenient spreadability and has a favourable safety and therapeutic profile. Microemulgel, despite facing challenges, has a strong potential to become the primary topical administration method for lipophilic drugs in the future.
This review focuses on the several types of microemulsion, their advantages over other dosage forms, how they are prepared, characterized, and employed in different industries.
Keywords: Microemulsion, Microemulgel, topical drug delivery, lipophilic, In vitro profile.
Introduction
It is believed that the human skin is a useful and accessible part of the body for topical treatments. An adult’s skin surface area is about 1.8 m2, including 200-300 sweat ducts and 40-70 hair follicles. The pH of the skin ranges from 4 to 5.6 based on the exudate produced by the sweat glands. Sweat ducts, the intact stratum corneum, and sebaceous glands are the three main entrance sites for molecules into the skin. Dead cells makeup the stratum corneum, the uppermost layer of the epidermis. As a strong water barrier, it shields the blood vesicles scattered under the skin’s surface as well as the deep internal components.
The lipid matrix of the stratum corneum is a stratified structure made up of various fatty acids, cholesterol, ceramides, and cholesteryl ester. A drug must overcome obstacles such as skin separation to deliver the right drug concentration to the desired area and enter the systemic circulation. A microemulgel will be the most effective solution for this purpose¹.
Throughout the history of conventional medicine, the human skin has been widely employed as the primary organ to distribute different medications and achieve the intended therapeutic effect.
The purpose of this action was to achieve the desired therapeutic effect. The Transdermal Drug Delivery System (TDDS), which has been a popular substitute for the oral route of drug administration in current medical practice for many years, has similarly made a significant contribution to health care. Patient drug administration can also be accomplished via the Transdermal Drug Delivery System (TDDS)².
Topical drug delivery methods provide various benefits, such as the ability to deliver medication more effectively and selectively to a particular area while avoiding the metabolic breakdown linked to systemic administration³.
A drug needs to get through these barriers in order to reach systemic circulation and the desired concentration at the site of action. For this requirement, a microemulsion gel with globule-shaped colloidal carriers would be the perfect formulation⁴.
DRUG DELIVERY THROUGH A TOPICAL ROUTE
The characteristics of the ideal formulations include patient compliance, self-administration, non-invasiveness, fewer side effects, and better pharmacological action. Topical route administration offers benefits such as avoiding hepatic first-pass effects, decreased side effects due to local action, enhanced percutaneous absorption and increased bioavailability with sustained deposition. It also reduces drug loss due to metabolism or decomposition and allows drug targeting at the desired site. Minimizing drug breakdown and constant delivery for a prolonged period results in prominent movement of the drug across the stratum corneum barrier, improving bioavailability⁵.
A drug may penetrate into the skin structure through-
I. thick stratum corneum, (SC)
II. sebaceous follicle.
III. sweat ducts of skin,
The stratum corneum covers over 99% of the skin, making it possible for medications to be absorbed. The drug's rate of percutaneous absorption is limited by passing through this stage. The development of a concentration gradient is one of the key processes in percutaneous absorption, which supplies the force required for drug adsorption through the skin⁶.

Table 1- Comparison between conventional Emulgel and Microemulgel⁵.
Parameter | Conventional emulgel | Microemulgel |
Thermodynamic stability | Not stable due to the natural tendency of coalescence that causes creaming or sedimentation | Stable-because of their smaller particle size, Brownian motion provides enough stability against gravity, avoiding sedimentation or creaming |
Particle size | Greater than>500nm | Less than 500nm |
Bioavailability | Comparatively less bioavailable than Nanoemulgel | Enhanced bioavailability, attributed to small size and large surface area |
Permeation | Comparatively lower permeation | High permeation owing to its lower particle size |
Preparation | Require high energy techniques | It can be prepared either by using high or low energy techniques |
Systemic absorption | Very minimal | Higher compared to conventional emulgel due to the small particle size and large surface area |
Ability to cross BBB | Cannot cross BBB | Can cross BBB because of its small particle size |
Nanotechnology is one of the rapidly growing technical applications that has been utilized more and more for a variety of purposes, particularly in the food, pharmaceuticals, and cosmetics sectors. Products including nanotechnology have a promising market because of their superior qualities, which include tiny droplet size with high interfacial area, improved active ingredient delivery, and great solubilization capability⁵.
MICROEMULGEL AS TOPICAL DRUG DELIVERY SYSTEM
The combination of the hydrogel and microemulsion systems is known as microemulgel. Both technologies have some drawbacks, such as the low spreadability and retention of the microemulsion and the inability of hydrogels to include lipophilic molecules⁶. With droplet sizes ranging from 5 to 500 nm, microemulgel contains a variety of polymeric components, surfactants, and fatty compounds of natural, synthetic, and semisynthetic origin. It is possible to get around both methods' limitations with microemulgel. In order to create microemulgel—which permits the integration of a lipophilic drug into a hydrogel while also increasing the viscosity of the microemulsion—the lipophilic drug is dissolved in the oil phase of the microemulsion, which is then added to the hydrogel basis⁷.
Microemulgel is used in transdermal medication delivery as a drug reservoir. The medication initially enters the outer phase, then moves into the skin's surface from the inner phase. Oily droplets are liberated from the gel matrix of the microemulgel when it is applied to the skin. These droplets pass through the stratum corneum to enter the skin deeply and deliver the drug moiety directly⁸. Both the crosslink density and the makeup of a network of polymer chains influence the drug release mechanism⁹.
MICROEMULSION
Microemulsion is a promising method for drug delivery, optimizing effectiveness and reducing toxicity. It consists of combining nano ranges of two immiscible liquids (water and oil) to form a homogeneous solution, with appropriate surfactants or cosurfactants. The stable, thermodynamic system ranges from 10-100 nm. A microemulsion enhances drug delivery by targeting poorly soluble drugs, increasing absorption through the skin, improving drug processing time, and reducing side effects¹⁰. The effects of microemulsion with nano-scale globules do not affect the emulsion's physical properties. Research shows that lacidipine bioavailability via transdermal route is 3.5 times higher than oral courses, due to avoidance of first-pass metabolism. Microemulsion also improves drug permeation across the skin, as the small size of particles allows more medication to be introduced into the mixture, enhancing thermodynamics towards the skin. The drug-affinity for partitioning also enhances skin permeation⁸.
![Figure-2 Structure of Microemulsion[9]](https://static.wixstatic.com/media/44d653_c133d05638dd4850907798ea91fdc4a7~mv2.png/v1/fill/w_592,h_268,al_c,q_85,enc_avif,quality_auto/44d653_c133d05638dd4850907798ea91fdc4a7~mv2.png)
MICROEMULGEL
Microemulgel is a gel base formation that contains microemulsion. It is created by incorporating a microemulsion system into a gel matrix, which improves skin penetration¹¹. The drug reservoirs in this mixture of microemulgel affect the drug's release from the inner phase to the outer phase and beyond. Oil droplets are released from microemulgel when skin is still intact. These droplets enter the skin's dermal capillaries and transport the medication to the desired location. The drug is more likely to penetrate the skin when applied in microemulsion-gel form because of its strong adhesion properties and high drug solubilization in the oil phase, which results in a greater concentration gradient. When compared to creams and ointments, the greater spreadability and lower stickiness also result in improved patient compliance¹².
Important components of a microemulgel:
A gel based microemulsion preparation for topical application comprises of specialized components apart from lipids and surfactants such as gelling agents, permeation enhancers, preservatives and antioxidants.
Aqueous phase
This element is responsible for transforming the emulsion into an emulgel. Generally ultra-purified water or distilled water is used for the production of a microemulgel¹.
Oily phase
The selection of oil or other lipid components must ensure that the oily phase is real and shielded from impurities such as peroxides, free radicals, and other fatty acids such as sterols and polymers. One of the primary factors considered when choosing lipids for the creation of microemulgels is the presence of most hydrocarbon chains; this decision is made with the fundamental principles of emulsification and consistency in mind. Mineral oil as a drug vehicle, cottonseed oil, maize oil, arachis oil, olive oil, coconut oil, eucalyptus oil, rose oil, clove oil, etc. are among the oils that are frequently employed in microemulsions.
Surfactants and co-surfactants
Due to the surfactant’s amphiphilic nature, two immiscible phases can disperse, lowering interfacial tension and producing a stable enough film to form around the droplets with the optimum curvature¹³. Surfactants are molecules that can improve the stratum corneum (SC) diffusion coefficient by reversibly attaching to keratin filaments, destroying corneocytes, and improving penetration through the skin¹⁴. Depending on the concentration of the surfactant combination, different medications have varying effects on skin penetration¹⁵. When the concentration of surfactant is increased, the permeation of hydrophilic drugs is significantly enhanced¹⁶.
Surfactants are employed during the microemulgel production process to give the final formulation stability and emulsification. Non-ionic surfactants are employed in the creation of microemulgel due to their low level of toxicity. Non-ionic surfactants that are often employed include polyoxyethylene fatty acid esters and sorbitan fatty acid ester³
Co-surfactants are typically employed to reduce the concentration of surfactant and improve the final product's thermodynamic stability. Transcutol HP, PEGs, glycerine, PGs, and ethyl alcohol are a few examples of co-surfactants¹.
An emulsion cannot be stabilized only by a co-surfactant. Rather, by enhancing surfactant action in a synergistic way, it helps generate microemulsions (MEs) and nanoemulsions (NEs). Interfacial tension will be further reduced in particular by a co-surfactant. Moreover, it enables increased oil penetration between the surfactant tails, supporting the ideal interfacial film curvature¹⁷.
Penetration enhancers
One of the greatest approaches to improve transportation efficiency through the skin and related layers has been to use penetration enhancers. One of the main components of the traditional drug delivery method, penetration enhancers are typically utilized in topical microemulgels. These penetration enhancers typically function by interacting with the constituents of the skin, resulting in a transient and cumulative elevation of skin permeability.
Gelling agent
One of the key components of microemulgel that provides the formulation its ideal structure is the gelling agent. These are cross-linking agents. Among the gelling agents that are employed are Carbopol, HPMC, and Tragacanth.
Preservatives
Preservatives are chemicals that are added to a product to prolong its shelf life by protecting the item from microbiological interference. Phenoxyethanol, benzalkonium chloride, methyl paraben, propyl paraben, and other preservatives are often employed.
Antioxidants
Antioxidants are chemical substances that are used in compositions to prevent oxidation of the different elements. Examples include butylated hydroxyl toluene, ascorbyl palmitate etc¹.
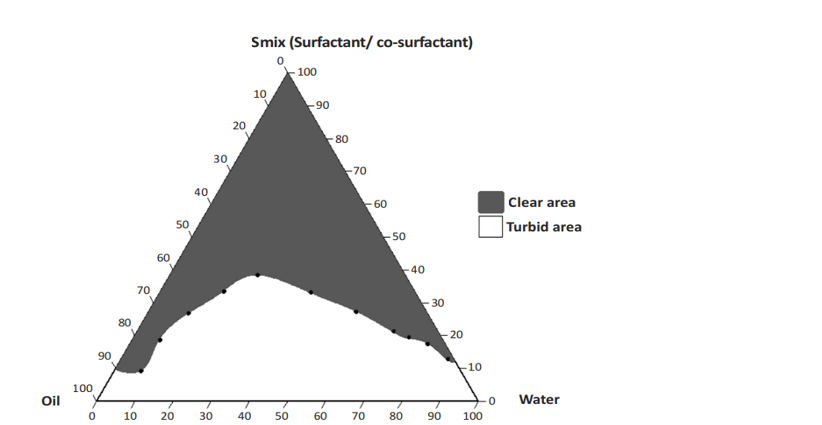
CONSTRUCTION OF A PSEUDOTERNARY PHASE DIAGRAM
A pseudoternary phase diagram is a tool that optimizes the three components of any typical emulsion, i.e., water, oil, and surfactant, to obtain the concentration range of these components, which form a stable emulsion. The three components of a system are plotted on the three corners of the triangle.
In this example surfactant and co-surfactant (Smix) were mixed in different ratios (2:1, 3:1 and 5:1). Each ratio was chosen in increasing amount of surfactant with respect to co-surfactant for a study of the phase diagram. Here, aqueous phase (distilled water) was used as dilution medium. Oil and Smix were mixed at different ratios from 9:1 to 1:9 in different vials for each Smix. The main objective for this is to cover for the study to decide the boundaries of phases formed in the diagrams. It was developed using a titration method with help of water as the aqueous medium. Slow titration of oil and Smix is performed and visual observations are made for transparency of the microemulsion.
One axis represents the aqueous phase of the microemulsion, another represents the oil phase, and the third represents the Smix (surfactant and co-surfactant) phase⁶
ADVANTAGES OF MICROEMULGELS
Microemulgel has various advantages over other topical formulations as well as conventional preparation, which are as follows-
Avoids first pass metabolism.
Easily accepted by the patient.
Appropriate for self-administration of medicines.
Provides for local delivery of drugs.
Simple medication discontinuation².
Easily adapted to the environment of the skin.
Proven efficacy for a controlled and sustained medication delivery system.
Microemulgels do not cause irritation or toxicity.
Improved medication loading as compared to other formulations.
Enhanced medication deposition and skin permeability⁶.
Offer a formulation with a higher spreadability than creams¹².
Limitations
In order to maintain a nanoscale particle, the method is expensive due to the requirement for a homogenizer¹⁹.
Enhanced drug permeability through skin
The permeability of the drug through the skin has been significantly improved by microemulgel compared to other formulations because, in microemulgel preparation, the drug can permeate the skin layer through both paracellular and transcellular routes, whereas in microemulsion, only the transcellular permeation route is observed. Figure 4 illustrates a comparison of cumulative medication permeability through the skin from various formulations⁹.

PREPARATION METHODS FOR MICROEMULSION
The oil phase or the aqueous phase both dissolve the selected surfactant. The medicine is then introduced and dissolved in the aqueous or oil phase, depending on its solubility, then heated. After that, the mixture is continuously stirred while one phase is progressively added until it reaches room temperature⁹.
Step 1: Preparation of microemulsion
A spontaneously formed microemulsion can be produced by adding high energy to the heterogeneous mixture or by mixing the compositions and reducing the interfacial tension between the oil/water interfaces. In order to create a thermodynamically stable microemulsion, both high-energy and low-energy emulsification techniques may be applied.
High-energy method
Since the average size of a microemulsion droplet is between 5 and 500 nm, a significant amount of mechanical energy is needed to achieve this size. Numerous methods, such as high-pressure homogenizers, ultrasonic generators, microfluidizers, and high-speed homogenizers, can be used to achieve high-energy input for manufacturing. The main advantage of utilizing a high-energy mediated microemulsion formulation is the utilization of low emulsifier concentrations. The first stage in using high-energy methods is the mechanical churning that creates an emulsion with droplet sizes in the micron range. The second phase involves using high-energy equipment to split large droplets into tiny droplets, which transforms the emulsion into a microemulsion²⁰.
Ultrasonication
The rough emulsion is transformed with a sonicator probe into desired nano-sized emulsion droplets. It is the sonicator probe that produces high-intensity sound waves with frequencies higher than 20 kHz. This is capable of breaking up the rough emulsion into droplets that are nanosized (5-500nm). Size reduction up to acceptable levels is possible with a variety of probe kinds and diameters. Droplet scale is dependent on time, probe type, and strength of sonication input.
High-pressure homogenization technique
In this technique, globule size can be lowered to the nanoscale range using a piston homogenizer or high-pressure homogenizer (microfluidizer). In the microfluidizer technique, impact, attrition, turbulence, and hydraulic shear are delivered in addition to an extremely high pressure of around 500–20,000 psi during the emulsification process. The macro emulsion is changed into a coarse emulsion by the combined action of many forces, including cavitation, shear, and hydraulic pressures. The product is then put through the same procedure to produce droplets with the appropriate size and Polydispersity Index (PDI). A crucial component of the ideal emulsification is the number of homogenization cycles. Minimal levels of surfactant are utilized in this procedure, therefore there is very little risk of contamination. The homogenizers in piston-type homogenization processes function according to the colloid mill principle. The coarse emulsion is sprayed into a gap smaller than 10μm in dimension during this process of creating nanosized droplets. Here in the piston, a stationary stator and a continuously revolving rotor operate on the coarse emulsion until, after several high-shear rotation cycles, the coarse emulsion transforms into the appropriate size droplets.
Solvent Evaporation Technique
This approach involves dissolving the medication in an appropriate phase to create an emulsion, which is then obtained by evaporating the drug. Particle aggregation and crystal development during the precipitation process can be managed with the use of a high-speed stirrer.
The transdermal microemulsion exhibits a number of characteristics that impact the topical contact duration of the microemulsion, including excellent drug solubility, thermodynamic stability, improved penetration ability, and low viscosity. Because of this, different gel matrices, such as carbomer 934, HMC, acacia, etc., are incorporated into the microemulsion, increasing its viscosity. Because of its dual control release and more viscous system, the microemulgel system—which is created by incorporating gel matrix into microemulsion—has a greater transdermal applicability than microemulsion. There are two ways to make the microemulgel: either by putting the gelling agent directly into the microemulsion or by adding it to the water phase first, forming a gel before adding it to the microemulsion. Both the O/W and W/O types of emulsion are used to make microemulgel.
Phase Inversion Method
A phase inversion technique is presented by Shinoda et al. Phase inversion in this system is achieved by varying component composition at constant temperature, which modifies the system's chemical energy and promotes the creation of a homogeneous emulsion²¹.
Sonication Method
Particle sizes in the dispersed phase are decreased throughout the sonication process by use of a sonicator. However, this procedure is limited to small-scale manufacturing²¹.
Low-energy method
Low-energy emulsification techniques use less energy than high-energy techniques to produce microemulsion. The system's natural chemical energy is used to create a microemulsion, and just gentle stirring is needed. Some low-energy methods are spontaneous emulsification and phase inversion techniques²⁰.
Spontaneous emulsification
Spontaneous emulsification is among the most practical techniques for producing a microemulsion. It consists of two liquid components: an organic component and an aqueous component. Water miscible solvents, surfactants, and co-surfactants are moved from the organic phase into the aqueous phase. The procedure begins with the introduction of an organic phase—such as oil and surfactant—into an aqueous phase, which is composed of co-surfactant and water. Rapid migration of water-miscible components into the aqueous phase raises the oil-water interfacial area and results in massive turbulence at the phase contact. Consequently, small oil droplets appear on their own²².
Step 2: Preparation of microemulgel
The polymer is dissolved in purified water and continuously stirred with a mechanical stirrer to create the gel basis. The gelling agent and the microemulsion are prepared, and then the two are continually mixed until a microemulgel forms. Using various polymeric gelling agents, water in oil (w/o) or oil in water (o/w) microemulsion may be transformed into thick, semisolid microemulgels⁹.
To produce the gel base, the necessary gelling ingredient is dissolved in distilled water whilst stirring continuously. To create the microemulgel, the pH of the prepared gel is adjusted, and then the microemulsion system is gradually added to the gel at a certain ratio while being stirred continuously⁹.
Preparation of gelling agent
The goal of using a gelling agent in the formation of a microemulgel is to change its physical status from liquid to semi-solid, which offers several benefits for patient compliance. In order to make different types of gel bases for gelling, the polymer can be added to purified water and continually agitated with a glass rod or any other appropriate mechanical device until the required texture is formed. Afterwards, the pH should be adjusted²³. The polymer is added to purified water using a cold approach in a variety of experimental operations to prepare the gelling agent. The cold technique involves adding the ingredients to filtered water at 200C, then adding the gelling polymer and cooling the water to 400C²⁴.
Incorporation of gelling agent
A microemulgel is produced by mixing the microemulsion and gelling agent once they have been manufactured. Here, several polymeric gelling agents are used to transform a liquefied version of water in oil (w/o) or oil in water (o/w) microemulsion into a thick and semisolid microemulgel. This gel form can be transformed back into a solution form by rubbing or using another mechanical force. This material's characteristic is called thixotropy, and it allows for the transformation of gel to sol and sol to gel with the application of shear stress and its reverse, respectively, without causing a change in volume. Several polymers, including methyl cellulose, carbomer 940, carbopol 943, chitosan, carbopol 934, and carbopol 940, have been employed as gelling agents to create microemulgel with the appropriate properties for a range of uses²⁵.
Gels based on Microemulsion are prepared by mixing 1g of gelling ingredient with enough distilled water. After placing this gelling agent solution in the dark for 24 hours, the entire swelling system is achieved. Next, while magnetic stirring is in place, the drug-loaded microemulsion is gradually added to the gelling agent's viscous solution¹².

EVALUATION OF MICROEMULGEL
1. Visual inspection: To determine the colour, appearance, and homogeneity of the produced microemulgel, visual inspection may be performed²⁶.
2. pH measurement: The pH of a Microemulgel varies according to its intended application, such as on the skin or other mucous membranes. For example, the pH of human skin ranges from 4.5 to 6.
3. Determination of viscosity: The gel's viscosity is essential for effective skin application. Comprehending gel's rheological behaviour is essential. The fluid's resistance to flowing is known as its viscosity; a higher viscosity is associated with a higher flow resistance. Fluids can be divided into two main categories: Newtonian systems and non-Newtonian systems. A higher viscosity fluid in Newtonian flow requires more force per unit area, or shear stress, to generate a given shear rate. At different shear rates, the viscosity in Newtonian flow remains constant. Unlike Newtonian fluid, non-Newtonian flow defies Newton's low viscosity and is unaffected by changes in shear rate²⁷.
4. Spreadability measurement: The medical efficacy of the suggested formulation will be determined by how well the topical medicine spreads. The ease with which a gel covers the affected area and the skin's application site is referred to as spreadability. Spreadability of a microemulgel is evaluated based on its "Slip" and "Drag" properties²⁸.
5. Droplet size measurement and polydispersity index (PDI): The dynamic light scattering (DLS) method is commonly used to calculate droplet size. The generated microemulsion's homogeneity of droplet size can be determined through the measurement of the polydispersity index (PDI)²⁹.
6. Zeta potential: The combination of microemulsion and a gelling agent in microemulgel allows for the formulation to take on an electrical charge due to the presence of several surface-active components³⁰.
7. Drug content: Drug content is a crucial factor that determines how much drug is overall present in prepared formulae; a higher drug content is associated with minimal drug loss during the production process³¹.
8. Accelerated stability study: The International Council for Harmonization's (ICH) guidelines should be followed when conducting an accelerated stability study. The formulations should be stored in the oven at 37±2, 45±2, and 60±2 degrees Celsius for three months³. To ascertain the medication content, microemulgel should be checked every two weeks using the proper analytical method. The stability is determined by observing changes in the gel's pH or drug deterioration²⁰.
9. In vitro release study: Drug release studies are conducted in vitro using a Franz diffusion cell, which has an effective diffusion area of 3.14 cm2 and a cell capacity of 15.5 ml. The diffusion cell’s donor and acceptor chambers are encased in an even layer of the microemulsion that is applied to the membrane to dissolve the medication, new phosphate- buffered saline (pH 5.5) is added to the receptor compartment and a magnetic stirrer is used to stir the receiving chamber. Samples (aliquots of 1.0 ml) are gathered at appropriate intervals. Following the appropriate dilution, UV-Vis is used to determine the drug concentration of the sample. The total amount of medication that has been released via the dialysis membrane is calculated¹².
APPLICATIONS OF MICROEMULSION
Microemulsion for oral route-
When poorly water-soluble drugs are administered orally, their solubility, absorption, and bioavailability are increased by the o/w microemulsion, which increases the drug's rate of dissolution and low bioavailability.
Microemulsion for ocular delivery-
To enhance the absorption of lipophilic medicines, such as erythromycin and pilocarpine, o/w microemulsion is utilized.
Microemulsion for nasal delivery-
When considering the nasal route in comparison to the perioral and parenteral routes, there are numerous advantages. For example, the nasal mucosa has more time to come into contact with the microemulsion droplet, which increases drug absorption, and the nasal route bypasses the liver's initial processing.
Microemulsion for transdermal delivery-
The chemical can penetrate the skin in three different ways: through sweat ducts, hair follicles, or the stratum corneum itself. these routes regulate drug absorption and bioavailability, enhancing drug targeting and redistribution across blood and lymph arteries. The capacity of nano-sized emulsion to enter skin pores and achieve systemic administration makes it a viable technology with benefits including minimal preparation costs, good storage stability, and thermodynamic stability.
Microemulsion in cosmetics-
Microemulsion is considered to be an excellent carrier for delivering cosmetics in a controlled manner and helps disperse active ingredients throughout the skin layer Because microemulsion does not sediment, cream, or flocculate, it is utilized in cosmetics³².
CURRENT AND FUTURE PROSPECTS OF MICROEMULGEL
As hydrophobic drugs are poorly soluble and bioavailable, developing formulations for them has proven difficult. Because of their hydrophobic oleaginous bases, topical formulations such as creams, ointments, and lotions have good emollient qualities but sluggish drug release. Topical aqueous-based formulations that provide an aqueous environment, such as gels, improve medication release. Oily bases and hydrophobic APIs are combined to create an emulgel, which is then nanonized to create a microemulgel with improved characteristics. Microemulgels are a great dose form because they provide sustained release, improved permeability, and thermodynamic stability. The process of creating nano-emulsions restricts their commercialization even with its benefits. But as technology advances, it may eventually be possible to create manufacturing processes that are both profitable and commercially viable. Because nano-emulgel has advantages over conventional formulations, there has been a massive surge in production of nano-emulgel²⁰.
Microemulgel is an integral part of the topical delivery system. The following are some of the several applications for microemulgel in topical delivery:
Topical microemulsion gel is a better option than conventional lipophilic drug formulations due to its improved pharmacokinetic profile, longer half-life, and increased therapeutic efficacy. Comparing the microemulgel formulation to other topical administration options, one of the primary factors contributing to its higher patient approval is its better spreading properties and reduced stickiness. Problems associated with standard emulsions, such as creaming and phase separation, are resolved and spreadability is enhanced by including a microemulsion into the gel matrix. A gel filled with microemulsion is more advantageous in certain topical situations.
Topical Microemulgels are a more convenient and effective way to provide medication. Because the gel doesn't have an oily foundation, it releases medication more rapidly and has a greater patient compliance rate than other formulations. It is also non-greasy.
In the future, administering hydrophobic drugs may be accomplished more effectively and consistently with formulations based on microemulsion-gel. Many medications used to treat skin infections have hydrophobic properties. These medications can be effectively administered as microemulgels, in which the medicine is incorporated into the oil phase of the microemulsion before merging with the gel base. In spite of several obstacles, microemulgel is likely to be the mainstay for the topical administration of lipophilic medications in the future³³.
It has been discovered that microemulgel is a very effective delivery system for hydrophobic medications. With greater drug loading owing to higher solubilizing efficacy, improved bioavailability owing to superior permeability, and the ability to control drug release, it is a powerful alternative delivery technique for treating a variety of ailments. It has been shown that using microemulgel formulation to treat psoriasis, rheumatoid arthritis inflammation, fungal infections, acne, and pimples is far more successful³⁴. In addition to transdermal use, it may be utilized for ocular, vaginal, dental, and nose-to-brain pharmaceutical delivery for the treatment of many local and systemic disorders such alopecia, periodontitis, and Parkinson's disease. Microemulgel has been utilized as a UV absorber in the cosmetics industry³⁵.
Conclusion
Topical microemulgels have proven to be a more beneficial option for a dependable and useful medication delivery system. The gel-like texture and lack of grease make the formulation more patient-compliant, and the improved drug release results from not using oil as a foundation. Improved spreadability eliminates common emulsion issues such as creaming and phase separation, and microemulsion-gel compositions may provide a more effective and reliable method of administering hydrophobic drugs. Hydrophobic medications are frequently used to treat skin infections. By first being incorporated into the oil phase of the microemulsion and then combining with the gel base, these medications can be efficiently administered as microemulgels. Even with a few challenges, in the future, microemulgel is likely to be the primary topical delivery method for lipophilic medications. It provides multiple delivery options for topical drugs used to treat various conditions, such as high drug loading due to enhanced solubilizing efficiency and the capacity to modify drug release.
Stronger drug loading because of increased solubilizing effectiveness, enhanced bioavailability because of increased permeability, and the capacity to regulate drug release make it a powerful substitute delivery method for treating a wide range of local and systemic illnesses.
References
1. Verma, V. (2021). Microemulgel: Revolutionary approach for local geloriented formulation. IP International Journal ofcomprehensive and Advanced Pharmacology,6(1), 28-30.
2. Mandal, S., & Vishvakarma, P. (2023). Microemulgel: A Smarter Topical Lipidic Emulsion-based Nanocarrier. Indian J of Pharmaceutical Education and Research, 57(3s), s481-s498.
3. Azeez, A. R., & Alkotaji, M. (2022). Microemulgel as a recent drug delivery system. Military Medical Science Letters/Vojenské Zdravotnické Listy, 91(2).
4. Anand, K., Ray, S., Rahman, M., Shaharyar, A., Bhowmik, R., Bera, R., & Karmakar, S. (2019). Nano-emulgel: emerging as a smarter topical lipidic emulsion-based nanocarrier for skin healthcare applications. Recent patents on anti-infective drug discovery, 14(1), 16-35.
5. Donthi, M. R., Munnangi, S. R., Krishna, K. V., Saha, R. N., Singhvi, G., & Dubey, S. K. (2023). Microemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics, 15(1), 164.
6. 6.Begur, M. (2015). Enhanced permeability of cyclosporine from a transdermally applied microemulgel. Der Pharmacia Sinica; 6:69-79
7. Eid, A. M., El-Enshasy, H. A., Aziz, R., & Elmarzugi, N. A. (2014). Preparation, characterization and anti-inflammatory activity of Swietenia macrophylla microemulgel. J Nanomed Nanotechnol, 5(2),1-10.
8. Mou, D., Chen, H., Du, D., Mao, C., Wan, J., Xu, H., & Yang, X. (2008). Hydrogel-thickened microemulsion system for topical delivery of lipophilic drugs. International Journal of Pharmaceutics, 353(1-2), 270-276.
9. Sultana, N., Akhtar, J., Khan, M. I., Ahmad, U., Arif, M., Ahmad, M., & Upadhyay, T. (2022). Microemulgel: For Promising Topical and Systemic Delivery. In Drug Development Life Cycle. IntechOpen.
10. Shaji, K. P., Umesha, S., & Salimath, B. P. (2015). A novel liquid oral formulation for 1-Octacosanol an anticancer drug and its stability study. International Journal of Pharmacy and Analytical Research, 4(3)
11. Singh, R. P., Parpani, S., Narke, R., & Chavan, R. (2014). Emulgel: A recent approach for topical drug delivery system. Asian Journal of Pharmaceutical Research and Development, 112-123.
12. Jivani, M., Patel, C., & Prajapati, B. (2018). Microemulgel Innovative Approach for Topical Gel Based Formulation. Research dan Reviews on Healthcare Open Access Journal 1, 18–23.
13. Muzaffar, F. A. I. Z. I., Singh, U. K., & Chauhan, L. (2013). Review on microemulsion as futuristic drug delivery. Int J Pharm Pharm Sci, 5(3), 39-53.
14. Gupta, A., Eral, H. B., Hatton, T. A., & Doyle, P. S. (2016). Microemulsions: formation, properties and applications. Soft matter, 12(11), 2826-2841.
15. Kawakami, K., Yoshikawa, T., Moroto, Y., Kanaoka, E., Takahashi, K., Nishihara, Y., & Masuda, K. (2002). Microemulsion formulation for enhanced absorption of poorly soluble drugs: I. Prescription design. Journal of Controlled Release, 81(1-2), 65-74.
16. Hosmer, J., Reed, R., Bentley, M. V. L., Nornoo, A., & Lopes, L. B. (2009). Microemulsions containing medium-chain glycerides as transdermal delivery systems for hydrophilic and hydrophobic drugs. Aaps Pharmscitech, 10, 589-596.
17. Flanagan, J., & Singh, H. (2006). Microemulsions: a potential delivery system for bioactives in food. Critical reviews in food science and nutrition, 46(3), 221-237.
18. Vispute, S. (2021). Review on Nano-Emulgel. International Journal of Pharmacy & Pharmaceutical Research, vol22(2):367-376.
19. Paliwal, S., Pandey, K., Joshi, H., Kaur, G., & Akbar, N. An overview of microemulgel as a nanocarrier drug delivery.
20. Vazir, A. ed; (2023). Microemulgel- For promising topical and systemic delivery. Int J. Pharm.drug .Anal, vol:11, Issue:4, 2023;8-13.
21. Harshitha, V., Swamy, M. V., Kumar, D. P., Rani, K. S., & Trinath, A. (2020). Microemulgel: A process promising in drug delivery system. Research Journal of Pharmaceutical Dosage Forms and Technology, 12(2), 125-130.
22. Saka, R., Jain, H., Kommineni, N., Chella, N., & Khan, W. (2020). Enhanced penetration and improved therapeutic efficacy of bexarotene via topical liposomal gel in imiquimod induced psoriatic plaque model in BALB/c mice. Journal of Drug Delivery Science and Technology, 58, 101691.
23. Sengupta, P., & Chatterjee, B. (2017). Potential and future scope of microemulgel formulation for topical delivery of lipophilic drugs. International journal of pharmaceutics, 526(1-2), 353-365.
24. Anand, K., Ray, S., Rahman, M., Shaharyar, A., Bhowmik, R., Bera, R., & Karmakar, S. (2019). Nano-emulgel: emerging as a smarter topical lipidic emulsion-based nanocarrier for skin healthcare applications. Recent patents on anti-infective drug discovery, 14(1), 16-35.
25. Elosaily GH. Formulation and in vitro evaluation of nystatin microemulsion-based gel for topical delivery. J Am Sci 2012; 2012(8): 541-8.
26. Singh, G., Singh, D., Choudhari, M., Kaur, S. D., Dubey, S. K., Arora, S., & Bedi, N. (2021). Exemestane encapsulated copolymers L121/F127/GL44 based mixed micelles: Solubility enhancement and in vitro cytotoxicity evaluation using MCF-7 breast cancer cells. Journal of pharmaceutical investigation, 51, 701-714.
27. Saka, R., Jain, H., Kommineni, N., Chella, N., & Khan, W. (2020). Enhanced penetration and improved therapeutic efficacy of bexarotene via topical liposomal gel in imiquimod induced psoriatic plaque model in BALB/c mice. Journal of Drug Delivery Science and Technology, 58, 101691.
28. Pandi, P., Jain, A., Kommineni, N., Ionov, M., Bryszewska, M., & Khan, W. (2018). Dendrimer as a new potential carrier for topical delivery of siRNA: A comparative study of dendriplex vs. lipoplex for delivery of TNF-α siRNA. International journal of pharmaceutics, 550(1-2), 240-250.
29. KC, S., Kakoty, V., Krishna, K. V., Dubey, S. K., Chitkara, D., & Taliyan, R. (2021). Neuroprotective efficacy of co-encapsulated rosiglitazone and vorinostat nanoparticle on streptozotocin induced mice model of Alzheimer disease. ACS Chemical Neuroscience, 12(9), 1528-1541.
30. Sakamoto, K., Lochhead, R. Y., Maibach, H. I., & Yamashita, Y. (Eds.). (2017). Cosmetic science and technology: theoretical principles and applications. Elsevier.
31. Tiwari, N., Osorio‐Blanco, E. R., Sonzogni, A., Esporrín‐Ubieto, D., Wang, H., & Calderón, M. (2022). Nanocarriers for skin applications: where do we stand?. Angewandte Chemie International Edition, 61(3), e202107960.
32. Sadeq, Z. A. (2020). Review on microemulsion: Preparation and evaluation. International Journal of Drug Delivery Technology, 10(1), 187-189.
33. Mishra, P., Handa, M., Ujjwal, R. R., Singh, V., Kesharwani, P., & Shukla, R. (2021). Potential of nanoparticulate based delivery systems for effective management of alopecia. Colloids and Surfaces B: Biointerfaces, 208, 112050.
34. Wöll, S., Schiller, S., Bachran, C., Swee, L. K., & Scherließ, R. (2018). Pentaglycine lipid derivates–rp-HPLC analytics for bioorthogonal anchor molecules in targeted, multiple-composite liposomal drug delivery systems. International journal of pharmaceutics, 547(1-2), 602-610.
35. Sharma, D.R. babu (2023). Microemulgel A Novel Approach topical delivery system- updated Review. International journal of a drug development and research 0975-9344. vol.15 No 1- 988.
Author Information
Authors: Gupta Ashish¹, Darwhekar Gajanan¹, Jain Sachin Kumar²
¹Acropolis Institute of Pharmaceutical Education and Research Indore MP, India-453771
²Institute of Pharmacy, Oriental University Indore MP, India-453555
Corresponding Author:
Gupta Ashish, Acropolis Institute of Pharmaceutical Education and Research Indore MP, India-453771



Comments