- lauraclark849
- Dec 9, 2025
- 9 min read
Updated: Dec 19, 2025
Peer Review Article | Open Access | Published 18th December 2025
Feasibility assessment of recombinant reagents for the application of endotoxin testing of pharmaceutical waters.
P. Cliffe¹ K. Capper*² | EJPPS | 304 (2025) |https://doi.org/10.37521/ejpps30402
Abstract
The traditional Limulus amoebocyte lysate (LAL) assay has served as the standard method for detecting bacterial endotoxins in pharmaceutical waters; however, it relies on proteins derived from horseshoe crab blood, raising both ecological and ethical concerns. This study presents a comparative feasibility assessment of animal-free, synthetic (recombinant) reagents, specifically Recombinant Factor C (rFC) and Recombinant Cascade Reagent (rCR), for endotoxin detection. Reagents from five commercial suppliers were evaluated alongside conventional LAL-based assays. Both recombinant and traditional methods were assessed for their sensitivity and applicability in pharmaceutical water matrices. Our findings contribute to the ongoing transition toward sustainable, animal-free quality control practices in the pharmaceutical industry.
Introduction
Endotoxin contamination in pharmaceutical water systems represents a significant risk to product safety. The LAL assay, based on the blood extract of the horseshoe crab (Limulus polyphemus), has long been the reference method for endotoxin detection. However, the method's reliance on animal-derived reagents has prompted the development of recombinant alternatives, which offer the potential for improved sustainability and ethical compliance.
Recent advances have enabled the production of synthetic (recombinant) cascade components, namely Recombinant Factor C (rFC) and Recombinant Cascade Reagent (rCR) that mimic the LAL cascade (Figure 1). rFC relies solely on recombinant Factor C to detect endotoxin, converting a fluorogenic substrate into a measurable fluorescent signal. In contrast, rCR replicates the entire cascade using recombinant Factor C, Factor B, and proclotting enzyme, with resulting chromogenic output analogous to the traditional LAL test. In this study, we evaluate the efficacy and feasibility of recombinant-based assays across products from multiple commercial suppliers, benchmarking performance against the conventional LAL assay.

Materials & Methods
Reagents and Test Systems
Recombinant reagents rFC and rCR were sourced from five commercial suppliers shown in Table 1. The traditional LAL assay served as the comparator, implementing the three-step cascade leading to chromogenic substrate cleavage and quantification at 405 nm.
Sample Collection and Preparation
LAL reagent water (LRW) was used to represent pharmaceutical water samples during feasibility assessment. LRW was selected over samples from on-site water systems to allow for testing consistency across all supplier’s reagents.
Analytical Procedures
All assays were performed following the manufacturers' instructions for optimal sensitivity and reproducibility. Standard curves were established using reference standard endotoxin (RSE). Results from recombinant and traditional assays were compared in terms of:
Sensitivity (limit of detection)
Specificity for lipopolysaccharide (LPS) via endotoxin spike recovery at different concentrations.
Suitability for water testing
Positive Product Control (PPC) recovery
Precision (sample, PPC and standard curve)
Ruggedness across multiple testers
Table 1. Summary of recombinant and traditional LAL-based reagents from five suppliers that were assessed as part of this feasibility study.
Supplier (anonymised) | Reagent type | Label |
Supplier 1 | Recombinant Factor C | rFC1 |
Supplier 2 | Recombinant Factor C | rFC2 |
Supplier 2 | Kinetic Chromogenic LAL | KCL1 |
Supplier 3 | Recombinant Cascade Reagent | rCR1 |
Supplier 3 | Kinetic Chromogenic LAL | KCL2 |
Supplier 4 | Recombinant Cascade Reagent | rCR2 |
Supplier 4 | Kinetic Chromogenic LAL | KCL3 |
Supplier 5 | Recombinant Cascade Reagent | rCR3 |
Supplier 5 | Kinetic Chromogenic LAL | KCL4 |
Supplier 4 | Recombinant Cascade Reagent | rCR4 |
Supplier 4 | Kinetic Chromogenic LAL | KCL5 |
Experimental design
RSE was prepared at 2000 EU/mL in LAL Reagent Water (LRW) and stored refrigerated for up to 14 days. To generate standard curves (5, 0.5, 0.05, and 0.005 EU/mL), RSE was thoroughly vortexed for 3 minutes prior to the first dilution and for 1 minute between each subsequent dilution. Working samples were prepared by diluting RSE in LRW as described above to concentrations of 0.5 or 0.005 EU/mL. LRW alone was also included. Samples/standards were vortexed for 1 minute before addition to either microplate or cartridge wells.
Samples and standard curves were loaded into the microplate and PPCs were prepared by spiking 5 EU/mL standard to yield 0.5 EU/mL in PPC wells. Plates were pre-warmed to 37°C when specified. Samples were added directly to cartridges before testing, for cartridge-based methods. Reagents or LAL were freshly prepared and added to samples, after which plates were placed immediately into the reader to initiate the assay. Sample-reagent pH was tested after assay completion.
Assay acceptance required post-reaction pH between 6 and 8, standard curve correlation coefficient ≥0.980, PPC recoveries within 50–200%, CV for each sample ≤25%, and negative controls registering below the lowest standard. Data analysis was performed using Microsoft Excel and GraphPad Prism v10.1.2. Statistical analysis was performed using One-way ANOVA with Multiple Comparisons to determine statistical significance. Where endotoxin concentration was reported as <0.005, a value of 0.005 has been used for graphical and analytical purposes. These values have been plotted at the limit of detection (LoD). Similarly, the LoD has been used as the ‘target concentration’ to calculate percentage endotoxin concentration for LRW samples.
Results & Discussion
Recovered endotoxin concentration
Samples containing 0.5 EU/mL
When LRW was spiked with 0.5 EU/mL, recovery between 0.274 and 0.623 EU/mL was seen. Recovered endotoxin concentration was significantly lower for rCR1 compared to rFC1 (p<0.01), rFC2 (p<0.01), and KCL3 (p<0.05). No other significant differences were seen. All sample-reagent post-assay pH results were within the acceptable criteria (pH 6-8).

Samples containing 0.005 EU/mL
Recovery of endotoxin in samples spiked with 0.005 EU/mL ranged from <0.005 to 0.010 EU/mL. The only significant difference seen was between rFC1 and rCR3 (p<0.05). The variation observed here was expected due to spiking at a concentration equal to that of the lowest standard. All sample-reagent post-assay pH results were within the acceptable criteria (pH 6-8).

LRW samples
LRW samples were reported to contain between <0.005 and 0.007 EU/mL. No significant difference was seen between any reagents or experiments. The results seen suggest a low-level contamination of water used, or wells tested, in rFC1 and rCR1 experiments. Despite testing the same lot of LRW throughout all experiments, bottles were changes once used and could explain low level contamination in some samples and not others. All sample-reagent post-assay pH results were within the acceptable criteria (pH 6-8).

Percentage recovery versus target concentration
Samples containing 0.5 EU/mL
Percentage recovery for samples spiked with 0.5 EU/mL versus the target spike concentration (0.5 EU/mL) ranged from 54.8% to 124.6%. Percentage recovery by rCR1 was significantly lower than rFC1 (p<0.01) and KCL3 (p<0.01). No other significant differences were seen. Recovery by all reagents falls within 50-200% of the expected endotoxin concentration. All sample-reagent post-assay pH results were within the acceptable criteria (pH 6-8).

Samples containing 0.005 EU/mL
Percentage endotoxin recovery of samples spiked with 0.005 EU/mL versus the target spike concentration (0.005 EU/mL) ranged from 100% to 200%. Percentage recovery by rFC1 was significantly higher than rCR3 (p<0.05). As mentioned previously, increased variation was expected when spiking LRW with such a low concentration of endotoxin. This is reflected in the variation in percentage recovery seen here. Endotoxin recovery shown here remains within 50-200% of the expected value (two data points for rFC1 reported as 200% recovery). All sample-reagent post-assay pH results were within the acceptable criteria (pH 6-8).

LRW samples
No significant difference was seen between the percentage endotoxin recovery in LRW samples. As LRW samples were not spiked, a “target” concentration of 0.005 EU/mL (LOD) was used to calculate percentage recovery. Recovery between 100 and 140% was seen. All sample-reagent post-assay pH results were within the acceptable criteria (pH 6-8).

PPC recovery
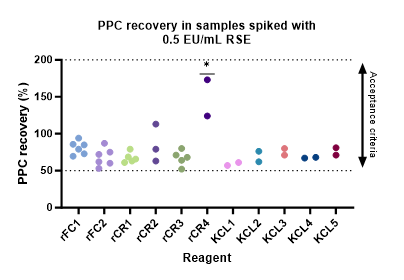
Samples containing 0.5 EU/mL
PPC recovery for samples containing 0.5 EU/mL ranged from 52% to 173%. This is within the 50-200% acceptance criteria; however, a several results fell below 70% which is lower than was expected. Low PPC recovery was investigated, and no experimental design/handling issues could be identified. It was suggested that reaction times of the standard curve for kinetic methods could influence the PPC recovery, which is a factor to track more closely in future studies. PPC recovery by rCR4 was significantly higher than all other reagents tested (p<0.05). It should be noted only two experiments with high variation have led to this statistic. All sample-reagent post-assay pH results were within the acceptable criteria (pH 6-8).
Samples containing 0.005 EU/mL
PPC recovery for samples spiked with 0.005 EU/mL ranged from 53% to 127%. Again, these values fall within the acceptance criteria however many remain lower than expected. PPC recovery by KCL3 was significantly higher than rCR1 (p<0.05), rCR3 (p<0.05) and KCL1 (p<0.05). All sample-reagent post-assay pH results were within the acceptable criteria (pH 6-8).

LAL reagent water samples
PPC recovery in LRW samples ranged from 55% to 142%. The low PPC recovery seen here was most concerning as it is assumed that LRW contains no factors that would interfere with endotoxin recovery.
Despite this, all results remained within the acceptance criteria. The only significance was seen between rCR4 and KCL5. All sample-reagent post-assay pH results were within the acceptable criteria (pH 6-8).

Discussion
Both rFC and rCR reagents demonstrated equivalent sensitivity to traditional LAL-based assays in the detection of endotoxin in pharmaceutical water samples. The rCR method, designed to recapitulate the three-enzyme cascade of the LAL test, displayed comparable kinetics and chromogenic signal output. The rFC-based assay, leveraging a fluorogenic readout, also performed reliably in the concentrations tested.
Qualitative and quantitative agreement with the LAL method was observed for most samples analysed. These findings suggest that recombinant assays serve as viable replacements for horseshoe crab-derived reagents, addressing both ethical considerations and sustainability goals within the industry.
It is noteworthy that the equivalence in performance across five different commercial suppliers indicates a degree of robustness and standardization within recombinant reagent manufacturing. However, ongoing validation in accordance with pharmacopeia guidelines and routine batch-to-batch consistency assessments remain necessary to ensure continued suitability.
Conclusion
This study demonstrates the feasibility of implementing recombinant endotoxin detection reagents for pharmaceutical water testing. Both rFC and rCR exhibited comparable sensitivity and performance to the reference LAL assay. Adoption of animal-free recombinant technologies stands to improve ethical standards and environmental sustainability, aligning endotoxin testing practices with evolving industry expectations.
Acknowledgements
Associates of Cape Cod, BioMérieux, Charles River Inc, Lonza, Fujifilm, Jill Hughes, Emily Butterworth, Caitlin Cooke, Ashleigh Jones.
References
USP <85> Bacterial Endotoxins Test
USP <86> Bacterial Endotoxins Test Using Recombinant Reagents
Ph. Eur. 2.6.14: Bacterial Endotoxins
Ph. Eur. 2.6.32: Test for Bacterial Endotoxins Using Recombinant Factor C
Appendix
Table 2. Average endotoxin recovery, percentage endotoxin recovery and PPC recovery across testing of LRW spiked with 0.5 EU/mL RSE by two testers. Values represent average ± standard deviation (SD).
Reagent | Endotoxin recovery (EU/mL) | Percentage endotoxin recovery (%) | PPC recovery (%) |
rFC2 | 0.473±0.037 | 94.5±7.4 | 68.2±12.3 |
rFC1 | 0.541±0.081 | 108.3±16.1 | 81.1±9.1 |
rCR4 | 0.365±0.076 | 72.9±15.1 | 148.5±34.6 |
rCR3 | 0.475±0.035 | 92.0±9.7 | 67.0±10.2 |
rCR2 | 0.411±0.066 | 82.3±13.3 | 85.0±25.5 |
rCR1 | 0.359±0.076 | 71.8±15.1 | 67.4±7.1 |
KCL5 | 0.417±0.116 | 83.4±23.2 | 76.0±7.1 |
KCL4 | 0.489±0.004 | 97.8±0.8 | 67.5±0.7 |
KCL3 | 0.579±0.015 | 115.8±3.1 | 75.5±6.4 |
KCL2 | 0.426±0.042 | 85.2±8.5 | 69.2±10.1 |
KCL1 | 0.451±0.006 | 90.3±1.2 | 59.0±2.8 |
Table 3. Average endotoxin recovery, percentage endotoxin recovery and PPC recovery across testing of LRW spiked with 0.005 EU/mL RSE by two testers. Values represent average ± standard deviation (SD).
Reagent | Endotoxin recovery (EU/mL) | Percentage endotoxin recovery (%) | PPC recovery (%) |
rFC2 | 0.005±0.00 | 103.8±5.0 | 88.5±22.0 |
rFC1 | 0.008±0.002 | 150.0±41.5 | 88.4±14.2 |
rCR3 | 0.005±0.00 | 101.3±2.1 | 73.5±6.9 |
rCR2 | 0.005±0.00 | 100.0±0.0 | 79.0±0.0 |
rCR1 | 0.006±0.002 | 116.7±32.0 | 75.3±14.2 |
KCL4 | 0.006±0.00 | 113.0±9.9 | 78.5±4.9 |
KCL3 | 0.005±0.00 | 100.0±0.0 | 116.5±14.8 |
KCL2 | 0.006±0.001 | 110.0±14.1 | 69.2±15.3 |
KCL1 | 0.005±0.00 | 100.0±0.0 | 63.5±9.2 |
Table 4. Average endotoxin recovery, percentage endotoxin recovery and PPC recovery across testing of LRW by two testers. Values represent average ± standard deviation (SD).
Reagent | Endotoxin recovery (EU/mL) | Percentage endotoxin recovery (%) | PPC recovery (%) |
rFC2 | 0.005±0.00 | 100.0±0.0 | 77.3±5.4 |
rFC1 | 0.006±0.001 | 113.3±16.3 | 90.8±15.8 |
rCR4 | 0.05±0.0 | 100.0±0.0 | 109.0±15.6 |
rCR3 | 0.005±0.00 | 100.0±0.0 | 78.5±8.2 |
rCR2 | 0.005±0.00 | 100.0±0.0 | 76.5±0.7 |
rCR1 | 0.005±0 | 103.3±8.2 | 81.6±18.5 |
KCL5 | 0.05±0.0 | 100.0±0.0 | 62.5±10.6 |
KCL4 | 0.005±0.00 | 100.0±0.0 | 84.0±11.3 |
KCL3 | 0.005±0.00 | 100.0±0.0 | 101.0±2.8 |
KCL2 | 0.005±0 | 100±0.0 | 77.3±8.8 |
KCL1 | 0.005±0.00 | 100.0±0.0 | 69.0±7.1 |
Authors
P. Cliffe¹ K. Capper*²
Corresponding Author: Karen Capper*²
AstraZeneca
Email: karen.capper@astrazeneca.com



Comments